Page 206 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 206
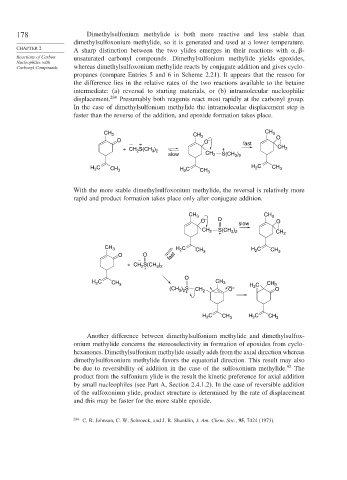
178 Dimethylsulfonium methylide is both more reactive and less stable than
dimethylsulfoxonium methylide, so it is generated and used at a lower temperature.
CHAPTER 2
A sharp distinction between the two ylides emerges in their reactions with -
Reactions of Carbon unsaturated carbonyl compounds. Dimethylsulfonium methylide yields epoxides,
Nucleophiles with
Carbonyl Compounds whereas dimethylsulfoxonium methylide reacts by conjugate addition and gives cyclo-
propanes (compare Entries 5 and 6 in Scheme 2.21). It appears that the reason for
the difference lies in the relative rates of the two reactions available to the betaine
intermediate: (a) reversal to starting materials, or (b) intramolecular nucleophilic
displacement. 284 Presumably both reagents react most rapidly at the carbonyl group.
In the case of dimethylsulfonium methylide the intramolecular displacement step is
faster than the reverse of the addition, and epoxide formation takes place.
CH 3 CH 3 CH 3
O O – O
– + fast
+ CH S(CH ) + CH 2
2
3 2
slow CH 2 S(CH )
3 2
H C CH 3 H C CH 3 H C CH 3
2
2
2
With the more stable dimethylsulfoxonium methylide, the reversal is relatively more
rapid and product formation takes place only after conjugate addition.
CH 3 CH 3
O – O O
slow
CH 2 S(CH ) CH
3 2
+ 2
CH 3 H C CH 3 H 2 C CH 3
2
O O fast
–
+ CH 2 S(CH 3 ) 2
+
O
H C CH 3 CH 3 H C CH 3
2
(CH ) S CH O – 2 O
3 2
+ 2
C CH H 2 C CH
H 2 3 3
Another difference between dimethylsulfonium methylide and dimethylsulfox-
onium methylide concerns the stereoselectivity in formation of epoxides from cyclo-
hexanones. Dimethylsulfonium methylide usually adds from the axial direction whereas
dimethylsulfoxonium methylide favors the equatorial direction. This result may also
be due to reversibility of addition in the case of the sulfoxonium methylide. 92 The
product from the sulfonium ylide is the result the kinetic preference for axial addition
by small nucleophiles (see Part A, Section 2.4.1.2). In the case of reversible addition
of the sulfoxonium ylide, product structure is determined by the rate of displacement
and this may be faster for the more stable epoxide.
284
C. R. Johnson, C. W. Schroeck, and J. R. Shanklin, J. Am. Chem. Soc., 95, 7424 (1973).