Page 203 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 203
The mechanistic details of reductive elimination reactions of this type are considered 175
in Section 5.8.
SECTION 2.4
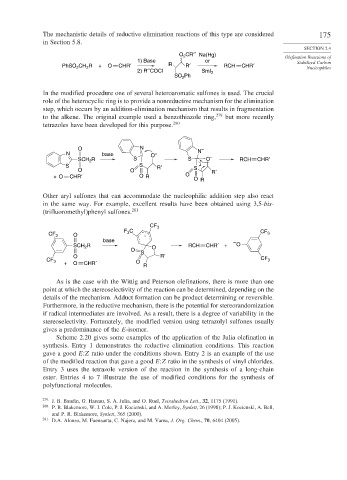
O CR′′ Na(Hg) Olefination Reactions of
2
1) Base or Stabilized Carbon
PhSO CH R + O CHR′ R R′ RCH CHR′ Nucleophiles
2
2
2) R′′COCl SmI 2
SO Ph
2
In the modified procedure one of several heteroaromatic sulfones is used. The crucial
role of the heterocyclic ring is to provide a nonreductive mechanism for the elimination
step, which occurs by an addition-elimination mechanism that results in fragmentation
to the alkene. The original example used a benzothiazole ring, 279 but more recently
tetrazoles have been developed for this purpose. 280
O N N –
N base O –
SCH R S S O – RCH CHR′
2
S S R′ S
O O R′
+ O CHR′ O R O
O R
Other aryl sulfones that can accommodate the nucleophilic addition step also react
in the same way. For example, excellent results have been obtained using 3,5-bis-
(trifluoromethyl)phenyl sulfones. 281
CF 3
F C
CF 3 O 3 - CF 3
base –
SCH R O RCH CHR′ + O
2
O
S
O R′
CF 3 O CF 3
+ O CHR′ R
As is the case with the Wittig and Peterson olefinations, there is more than one
point at which the stereoselectivity of the reaction can be determined, depending on the
details of the mechanism. Adduct formation can be product determining or reversible.
Furthermore, in the reductive mechanism, there is the potential for stereorandomization
if radical intermediates are involved. As a result, there is a degree of variability in the
stereoselectivity. Fortunately, the modified version using tetrazolyl sulfones usually
gives a predominance of the E-isomer.
Scheme 2.20 gives some examples of the application of the Julia olefination in
synthesis. Entry 1 demonstrates the reductive elimination conditions. This reaction
gave a good E:Z ratio under the conditions shown. Entry 2 is an example of the use
of the modified reaction that gave a good E:Z ratio in the synthesis of vinyl chlorides.
Entry 3 uses the tetrazole version of the reaction in the synthesis of a long-chain
ester. Entries 4 to 7 illustrate the use of modified conditions for the synthesis of
polyfunctional molecules.
279
J. B. Baudin, G. Hareau, S. A. Julia, and O. Ruel, Tetrahedron Lett., 32, 1175 (1991).
280 P. R. Blakemore, W. J. Cole, P. J. Kocienski, and A. Morley, Synlett, 26 (1998); P. J. Kocienski, A. Bell,
and P. R. Blakemore, Synlett, 365 (2000).
281
D.A. Alonso, M. Fuensanta, C. Najera, and M. Varea, J. Org. Chem., 70, 6404 (2005).