Page 202 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 202
174 Scheme 2.19. (Continued)
CHAPTER 2 13 l
Reactions of Carbon CH 3 O CH 3
Nucleophiles with CH 3 O CH 3 CH 3 H CH 3
Carbonyl Compounds H 1) –30°C
+ (C 2 H 5 ) 3 SiC CHN CHO
CHO 2) CF 3 CO 2 H, 0°C
Li
CH 3
CH 3 OTBDMS 91%
OTBDMS
a. K. Shimoji, H. Taguchi, H. Yamamoto, K. Oshima, and H. Hozaki, J. Am. Chem. Soc., 96, 1620 (1974).
b. P. A. Grieco, C. L. J. Wang, and S. D. Burke, J. Chem. Soc. Chem. Commun., 537 (1975).
c. I. Matsuda, S. Murata, and Y. Ishii, J. Chem. Soc., Perkin Trans. 1, 26 (1979).
d. F. A. Carey and A. S. Court, J. Org. Chem., 37, 939 (1972).
e. F. A. Carey and O. Hernandez, J. Org. Chem., 38, 2670 (1973).
f. D. Seebach, M. Kolb, and B.-T. Grobel, Chem. Ber., 106, 2277 (1973).
g. B. T. Grobel and D. Seebach, Chem. Ber., 110, 852 (1977).
h. P. Magnus and G. Roy, Organometallics, 1, 553 (1982).
i. W. Adam and C. M. Ortega-Schulte, Synlett, 414 (2003).
j. L. F. van Staden, B. Bartels-Rahm, J. S. Field, and N. D. Emslie, Tetrahedron, 54, 3255 (1998).
k. J.-M. Galano, G. Audran, and H. Monti, Tetrahedron Lett., 42, 6125 (2001).
l. S. F. Martin, J. A. Dodge, L. E. Burgess, and M. Hartmann, J. Org. Chem., 57, 1070 (1992).
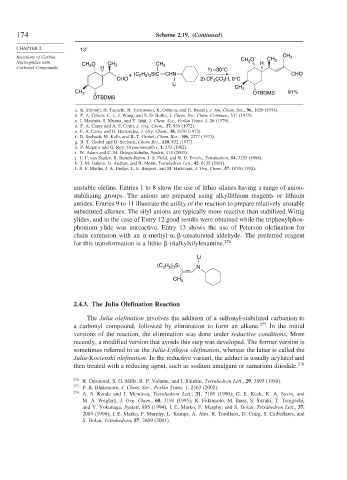
unstable olefins. Entries 1 to 8 show the use of lithio silanes having a range of anion-
stabilizing groups. The anions are prepared using alkyllithium reagents or lithium
amides. Entries 9 to 11 illustrate the utility of the reaction to prepare relatively unstable
substituted alkenes. The silyl anions are typically more reactive than stabilized Wittig
ylides, and in the case of Entry 12 good results were obtained while the triphenylphos-
phonium ylide was unreactive. Entry 13 shows the use of Peterson olefination for
chain extension with an -methyl- -unsaturated aldehyde. The preferred reagent
for this transformation is a lithio -trialkylsilylenamine. 276
Li
(C H ) Si N
2 5 3
CH 3
2.4.3. The Julia Olefination Reaction
The Julia olefination involves the addition of a sulfonyl-stabilized carbanion to
a carbonyl compound, followed by elimination to form an alkene. 277 In the initial
versions of the reaction, the elimination was done under reductive conditions. More
recently, a modified version that avoids this step was developed. The former version is
sometimes referred to as the Julia-Lythgoe olefination, whereas the latter is called the
Julia-Kocienski olefination. In the reductive variant, the adduct is usually acylated and
then treated with a reducing agent, such as sodium amalgam or samarium diiodide. 278
276
R. Desmond, S. G. Mills, R. P. Volante, and I. Shinkai, Tetrahedron Lett., 29, 3895 (1988).
277 P. R. Blakemore, J. Chem. Soc., Perkin Trans. 1, 2563 (2002).
278
A. S. Kende and J. Mendoza, Tetrahedron Lett., 31, 7105 (1990); G. E. Keck, K. A. Savin, and
M. A. Weglarz, J. Org. Chem., 60, 3194 (1995); K. Fukumoto, M. Ihara, S. Suzuki, T. Taniguchi,
and Y. Yokunaga, Synlett, 895 (1994); I. E. Marko, F. Murphy, and S. Dolan, Tetrahedron Lett., 37,
2089 (1996); I. E. Marko, F. Murphy, L. Kumps, A. Ates, R. Touillaux, D. Craig, S. Carballares, and
S. Dolan, Tetrahedron, 57, 2609 (2001).