Page 197 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 197
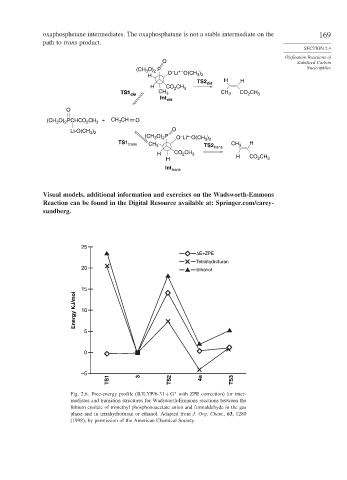
oxaphosphetane intermediates. The oxaphosphatane is not a stable intermediate on the 169
path to trans product.
SECTION 2.4
Olefination Reactions of
O Stabilized Carbon
(CH O) P – + – Nucleophiles
2
3
H O Li O(CH )
3 2
H H
TS2 cis
H CO CH 3
2
TS1TS1 ciscis CH 3 CH 3 CO CH 3
2
Int cis
O
(CH O) PCHCO CH 3 + CH CH O
3
2
2
3
Li-O(CH ) O
3 2
(CH O) P O Li O(CH )
–
+–
2
3
3 2
TS1 trans CH 3 TS2 trans CH 3 H
H CO 2 CH 3 H CO CH
H 2 3
Int trans
Visual models, additional information and exercises on the Wadsworth-Emmons
Reaction can be found in the Digital Resource available at: Springer.com/carey-
sundberg.
25
ΔE+ZPE
Tetrahydrofuran
20
Ethanol
15
Energy KJ/mol 10
5
0
–5 3
TS1 TS2 4a TS3
Fig. 2.6. Free-energy profile (B3LYP/6-31 + G with ZPE correction) for inter-
∗
mediates and transition structures for Wadsworth-Emmons reactions between the
lithium enolate of trimethyl phosphonoacetate anion and formaldehyde in the gas
phase and in tetrahydrofuran or ethanol. Adapted from J. Org. Chem., 63, 1280
(1998), by permission of the American Chemical Society.