Page 195 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 195
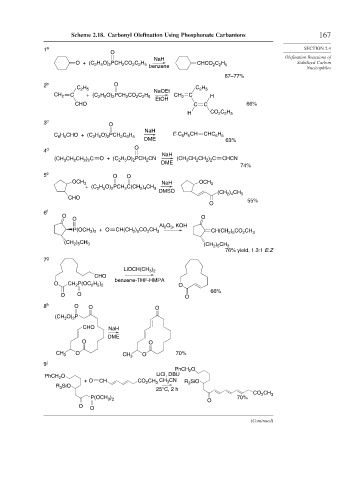
Scheme 2.18. Carbonyl Olefination Using Phosphonate Carbanions 167
1 a SECTION 2.4
O
Olefination Reactions of
NaH
O + (C H O) PCH CO C H benzene CHCO C H Stabilized Carbon
2 2 5
2
2
2 5
2 2 5
Nucleophiles
67–77%
2 b O
2 5
C 2 H 5 C H
NaOEt
CH 2 C + (C H O) PCH CO C H CH C H
2
2 2 5
2
2 5
EtOH 2
CHO C C 66%
C H
H CO 2 2 5
3 c O
NaH
C H CHO + (C H O) PCH C H E-C H CH CHC H
6 5
6 5
2
2 5
2 6 5
6 5
DME 63%
4 d O
NaH
H O) PCH CN
(CH CH CH ) C O + (C 2 5 2 2 (CH CH CH ) C CHCN
2 2
3
2
2
2 2
3
DME
74%
5 e O O
OCH 3 NaH OCH 3
+ (C H O) PCH C(CH ) CH 3
2
2 4
2
2 5
DMSO (CH ) CH
CHO 2 4 3 55%
O
6 f
O O
O
Al 2 O 3 , KOH
P(OCH 3 ) 2 + O CH(CH 2 ) 5 CO 2 CH 3 CH(CH ) CO CH 3
2 5
2
(CH ) CH 3 (CH 2 5 3
) CH
2 5
76% yield, 1.3:1 E:Z
7 g
)
LiOCH(CH 3 2
CHO
benzene-THF-HMPA
O CH P(OC H ) O
2 5 2
2
66%
O O O
8 h O O O
(CH O) P
3
2
CHO NaH
DME
O O
CH 3 O CH 3 O 70%
j
9
PhCH O
2
PhCH O LiCl, DBU
2
+ O CH CO CH CH CN R 3 SiO
3
3
2
R SiO
3
25°C, 2 h
CO CH 3
2
P(OCH ) O 70%
3 2
O O
(Continued)