Page 198 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 198
170 Another computational study included a solvation model. 267 Solvation strongly
stabilized the oxyanion adduct, suggesting that its formation may be rate and product
CHAPTER 2
determining under certain circumstances. When this is true, analysis of stereoselectivity
Reactions of Carbon must focus on the addition TS. Figure 2.6 shows the computed energy profile for
Nucleophiles with
Carbonyl Compounds the TSs and intermediates. TS1 is the structure leading to the oxyanion intermediate.
According to the energy profile, its formation is irreversible in solution and therefore
determines the product stereochemistry. The structure shows a rather small (30 –35 )
dihedral angle and suggests that steric compression would arise with a Z-substituent.
H H pro-E
O –
(CH O) P CO 2 CH 3
3
2
O
H pro-Z
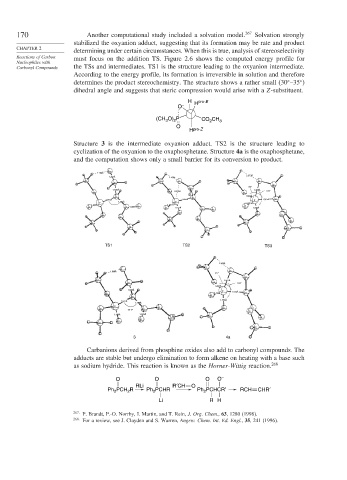
Structure 3 is the intermediate oxyanion adduct. TS2 is the structure leading to
cyclization of the oxyanion to the oxaphosphetane. Structure 4a is the oxaphosphetane,
and the computation shows only a small barrier for its conversion to product.
o
2.108Å
2.373Å
1.281Å 2.109A
c c o c o
c
c
130°
c
1.910Å 1.835Å 149°
o o 2.476A o
c c 1.694Å
1.791Å 1.858Å P 1.951Å c
P 1.461Å P 1.513Å
1.498Å 1.497Å o
o c 1.229Å o o 1.877Å c o 1.726Å
o c
o o o
o
c o o
c c
c c
c
TS1 TS2 TS3
2.365Å
c
1.956Å o
123°
c
c 2.861Å
c o 153°
1.686Å
1.665Å
P 1.932Å
o o 2.476Å c
c
2.837Å 1.733Å
1.488Å
P o
o c o c
111°
o
1.495Å 1.223Å c
c o
o
o
c
c
3 4a
Carbanions derived from phosphine oxides also add to carbonyl compounds. The
adducts are stable but undergo elimination to form alkene on heating with a base such
as sodium hydride. This reaction is known as the Horner-Wittig reaction. 268
O O O O –
RLi R′CH O
PCH R Ph PCHR Ph PCHCR′ RCH CHR′
Ph 2 2 2 2
Li R H
267 P. Brandt, P.-O. Norrby, I. Martin, and T. Rein, J. Org. Chem., 63, 1280 (1998).
268
For a review, see J. Clayden and S. Warren, Angew. Chem. Int. Ed. Engl., 35, 241 (1996).