Page 191 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 191
163
O Ph P CHOCH 3 CHOCH 3
3
SECTION 2.4
OCH OCH CH OCH 3 OCH OCH CH OCH 3
2
2
2
2
2
2
Olefination Reactions of
Stabilized Carbon
Ref. 248 Nucleophiles
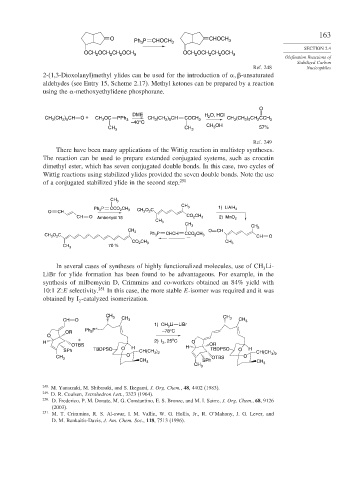
2-(1,3-Dioxolanyl)methyl ylides can be used for the introduction of -unsaturated
aldehydes (see Entry 15, Scheme 2.17). Methyl ketones can be prepared by a reaction
using the -methoxyethylidene phosphorane.
O
DME O, HCl
CH (CH ) CH O + CH 3 OC PPh 3 CH (CH ) CH COCH 3 H 2 CH 3 (CH ) CH CCH 3
2 5
3
2 5
2 5
2
3
–40°C
CH 3 CH 3 CH OH 57%
3
Ref. 249
There have been many applications of the Wittig reaction in multistep syntheses.
The reaction can be used to prepare extended conjugated systems, such as crocetin
dimethyl ester, which has seven conjugated double bonds. In this case, two cycles of
Wittig reactions using stabilized ylides provided the seven double bonds. Note the use
of a conjugated stabilized ylide in the second step. 250
CH 3
CH 3
Ph 3 P CCO 2 CH 3 CH 3 O 2 C 1) LiAlH 4
O CH
CH O Amberlyst 15 CO 2 CH 3 2) MnO 2
CH 3
CH 3
CH 3
O CH
CH 3
CH 3 O 2 C Ph 3 P CHCH CCO 2 CH 3
CH O
CO 2 CH 3 CH 3
70 %
CH 3
In several cases of syntheses of highly functionalized molecules, use of CH Li-
3
LiBr for ylide formation has been found to be advantageous. For example, in the
synthesis of milbemycin D, Crimmins and co-workers obtained an 84% yield with
10:1 Z:E selectivity. 251 In this case, the more stable E-isomer was required and it was
obtained by I -catalyzed isomerization.
2
CH CH
CH O 3 CH 3 3 CH 3
1) CH Li LiBr
3
Ph P +
OR 3 –78°C
O
+ o
H 2) I 2 , 25 C O
OTBS H OR
SPh TBDPSO O H CH(CH ) TBDPSO O H CH(CH )
3 2
CH O OTBS O 3 2
3
CH 3 SPh CH 3
CH 3
248
M. Yamazaki, M. Shibasaki, and S. Ikegami, J. Org. Chem., 48, 4402 (1983).
249 D. R. Coulsen, Tetrahedron Lett., 3323 (1964).
250 D. Frederico, P. M. Donate, M. G. Constantino, E. S. Bronze, and M. I. Sairre, J. Org. Chem., 68, 9126
(2003).
251
M. T. Crimmins, R. S. Al-awar, I. M. Vallin, W. G. Hollis, Jr., R. O’Mahony, J. G. Lever, and
D. M. Bankaitis-Davis, J. Am. Chem. Soc., 118, 7513 (1996).