Page 207 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 207
O CH 2 O 179
(CH ) C (CH ) C O +
3 3
(CH 3 ) 3 C
3 3
CH 2
SECTION 2.5
– +
S(CH ) THF Reactions Proceeding by
ylide: CH 2 3 2 83% 17%
0°C Addition-Cyclization
O
–
THF
ylide: CH 2 S(CH 3 ) 2 not formed only product
65°C
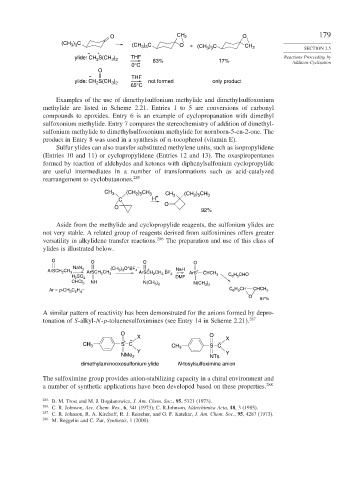
Examples of the use of dimethylsulfonium methylide and dimethylsulfoxonium
methylide are listed in Scheme 2.21. Entries 1 to 5 are conversions of carbonyl
compounds to epoxides. Entry 6 is an example of cyclopropanation with dimethyl
sulfoxonium methylide. Entry 7 compares the stereochemistry of addition of dimethyl-
sulfonium methylide to dimethylsulfoxonium methylide for nornborn-5-en-2-one. The
product in Entry 8 was used in a synthesis of -tocopherol (vitamin E).
Sulfur ylides can also transfer substituted methylene units, such as isopropylidene
(Entries 10 and 11) or cyclopropylidene (Entries 12 and 13). The oxaspiropentanes
formed by reaction of aldehydes and ketones with diphenylsulfonium cyclopropylide
are useful intermediates in a number of transformations such as acid-catalyzed
rearrangement to cyclobutanones. 285
(CH ) CH
CH 3 2 5 3 + CH 3 (CH ) CH 3
2 5
C H
O
O
92%
Aside from the methylide and cyclopropylide reagents, the sulfonium ylides are
not very stable. A related group of reagents derived from sulfoximines offers greater
versatility in alkylidene transfer reactions. 286 The preparation and use of this class of
ylides is illustrated below.
O O O O
+
NaN (CH ) O BF –
ArSCH CH 3 ArSCH CH 3 3 4 + – NaH + –
2 3 2 3 ArSCH CH BF 4 ArS CHCH C H CHO
2
3
H SO DMF 3 6 5
2 4
CHCl NH N(CH )
3 3 2 N(CH )
3 2
Ar = p-CH C H – C 6 H 5 CH CHCH 3
3 6 4
O
67%
A similar pattern of reactivity has been demonstrated for the anions formed by depro-
tonation of S-alkyl-N-p-toluenesulfoximines (see Entry 14 in Scheme 2.21). 287
O O
X X
+ – –
CH 3 S C CH 3 S C
Y Y
NMe 2 NTs
dimethylaminooxosulfonium ylide N-tosylsulfoximine anion
The sulfoximine group provides anion-stabilizing capacity in a chiral environment and
a number of synthetic applications have been developed based on these properties. 288
285
B. M. Trost and M. J. Bogdanowicz, J. Am. Chem. Soc., 95, 5321 (1973).
286
C. R. Johnson, Acc. Chem. Res., 6, 341 (1973); C. R.Johnson, Aldrichimica Acta, 18, 3 (1985).
287 C. R. Johnson, R. A. Kirchoff, R. J. Reischer, and G. F. Katekar, J. Am. Chem. Soc., 95, 4287 (1973).
288
M. Reggelin and C. Zur, Synthesis, 1 (2000).