Page 204 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 204
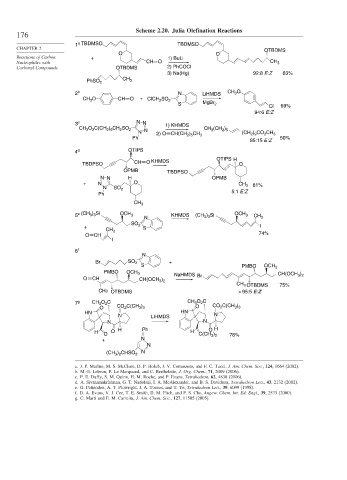
Scheme 2.20. Julia Olefination Reactions
176
1 TBDMSO TBDMSO
a
CHAPTER 2 OTBDMS
O O
Reactions of Carbon + 1) BuLi
Nucleophiles with CH O CH 3
Carbonyl Compounds OTBDMS 2) PhCOCl
3) Na(Hg) 92:8 E:Z 63%
CH 3
PhSO 2
2 b N LiHMDS CH 3 O
CH 3 O CH O + ClCH 2 SO 2
S MgBr 2
Cl 69%
94:6 E:Z
3 c N N 1) KHMDS
CH 3 O 2 C(CH 2 ) 9 CH 2 SO 2 N CH 3 (CH 2 ) 5
N (CH 2 ) 9 CO 2 CH 3
2) O CH(CH 2 ) 5 CH 3
Ph 50%
85:15 E:Z
4 d OTIPS
OTIPS H
KHMDS
TBDPSO CH O O
OPMB TBDPSO
N N H OPMB
+ N O CH 3 81%
N SO 2
Ph 5:1 E:Z
CH 3
5 e (CH 3 ) 3 Si OCH 3 KHMDS (CH 3 ) 3 Si OCH 3
N CH 3
SO 2 I
+ S
CH 3
O CH 74%
I
6 f
N
Br SO 2 +
S PMBO OCH 3
PMBO OCH 3
NaHMDS Br CH(OCH 3 ) 2
O CH CH(OCH 3 ) 2
CH 3 OTBDMS 75%
CH3 OTBDMS > 95:5 E:Z
7 g CH 3 O 2 C CH 3 O 2 C
O CO 2 C(CH 3 ) 3 O CO 2 C(CH 3 ) 3
HN HN
N LiHMDS N
N N
O H
H O O H Ph H C(CH 3 ) 2 78%
+ N
N
N
(CH 3 ) 2 CHSO 2
a. J. P. Marino, M. S. McClure, D. P. Holub, J. V. Comasseto, and F. C. Tucci, J. Am. Chem. Soc., 124, 1664 (2002).
b. M.-E. Lebrun, P. Le Marquand, and C. Berthelette, J. Org. Chem., 71, 2009 (2006).
c. P. E. Duffy, S. M. Quinn, H. M. Roche, and P. Evans, Tetrahedron, 62, 4838 (2006).
d. A. Sivaramakrishnan, G. T. Nadolski, I. A. McAlexander, and B. S. Davidson, Tetrahedron Lett., 43, 2132 (2002).
e. G. Pattenden, A. T. Plowright, J. A. Tornos, and T. Ye, Tetrahedron Lett., 39, 6099 (1998).
f. D. A. Evans, V. J. Cee, T. E. Smith, D. M. Fitch, and P. S. Cho, Angew. Chem. Int. Ed. Engl., 39, 2533 (2000).
g. C. Marti and E. M. Carreira, J. Am. Chem. Soc., 127, 11505 (2005).