Page 209 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 209
Scheme 2.21. (Continued) 181
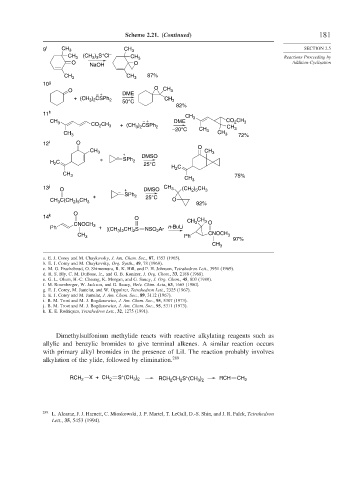
9 f CH 3 CH 3 SECTION 2.5
+
CH 3 (CH ) S Cl – CH 3 Reactions Proceeding by
3 3
O O Addition-Cyclization
NaOH
CH 3 CH 3 87%
10 g
O CH
O 3
– + DME
+ (CH ) CSPh 2 50°C CH 3
3 2
82%
11 h
CH 3
CH 3 CO CH – + DME CO CH 3
2
2
3
+ (CH 3 ) 2 CSPh 2
–20°C CH 3 CH 3
CH 3 CH 3 72%
12 i O
O
CH 3 CH 3
– + DMSO
+ SPh
H C 2 25°C
2
H C
2
CH 3 75%
CH 3
) CH
13 j O + DMSO CH 3 (CH 2 5
– SPh 3
+ 2 25°C
CH C(CH ) CH 3 O 92%
2 5
3
O
14 k O CH
CH 3 3 O
CNOCH 3
Ph + [(CH ) CH] S NSO Ar n-BuLi
2
3 2
2
CH 3 Ph CNOCH 3
97%
CH 3
a. E. J. Corey and M. Chaykovsky, J. Am. Chem. Soc., 87, 1353 (1965).
b. E. J. Corey and M. Chaykovsky, Org. Synth., 49, 78 (1969).
c. M. G. Fracheboud, O. Shimomura, R. K. Hill, and F. H. Johnson, Tetrahedron Lett., 3951 (1969).
d. R. S. Bly, C. M. DuBose, Jr., and G. B. Konizer, J. Org. Chem., 33, 2188 (1968).
e. G. L. Olson, H.-C. Cheung, K. Morgan, and G. Saucy, J. Org. Chem., 45, 803 (1980).
f. M. Rosenberger, W. Jackson, and G. Saucy, Helv. Chim. Acta, 63, 1665 (1980).
g. E. J. Corey, M. Jautelat, and W. Oppolzer, Tetrahedron Lett., 2325 (1967).
h. E. J. Corey and M. Jautelat, J. Am. Chem. Soc., 89, 3112 (1967).
i. B. M. Trost and M. J. Bogdanowicz, J. Am. Chem. Soc., 95, 5307 (1973).
j. B. M. Trost and M. J. Bogdanowicz, J. Am. Chem. Soc., 95, 5311 (1973).
k. K. E. Rodriques, Tetrahedron Lett., 32, 1275 (1991).
Dimethylsulfonium methylide reacts with reactive alkylating reagents such as
allylic and benzylic bromides to give terminal alkenes. A similar reaction occurs
with primary alkyl bromides in the presence of LiI. The reaction probably involves
alkylation of the ylide, followed by elimination. 289
+
+
RCH 2 X + CH 2 S (CH ) RCH CH S (CH ) RCH CH 2
3 2
3 2
2
2
289
L. Alcaraz, J. J. Harnett, C. Mioskowski, J. P. Martel, T. LeGall, D.-S. Shin, and J. R. Falck, Tetrahedron
Lett., 35, 5453 (1994).