Page 214 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 214
−
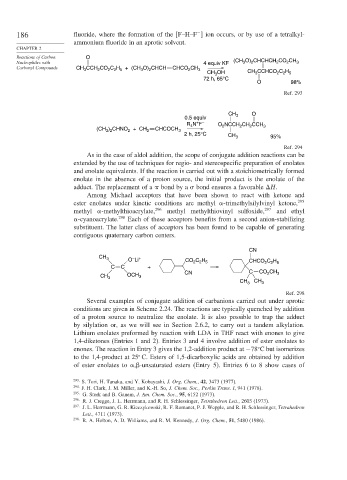
186 fluoride, where the formation of the
F–H–F ion occurs, or by use of a tetralkyl-
ammonium fluoride in an aprotic solvent.
CHAPTER 2
Reactions of Carbon O
2
3
2
2
Nucleophiles with 4 equiv KF (CH O) CHCHCH CO CH 3
Carbonyl Compounds CH CCH CO C H + (CH O) CHCH CHCO CH 3
2
2 2 5
3
2
2
3
CH OH CH CCHCO C H
2 2 5
3
3
72 h, 65°C
O 98%
Ref. 293
CH O
0.5 equiv 3
+ –
R N F O 2 NCCH CH CCH 3
4
2
2
(CH ) CHNO + CH 2 CHCOCH 3
3 2
2
2 h, 25°C CH 3 95%
Ref. 294
As in the case of aldol addition, the scope of conjugate addition reactions can be
extended by the use of techniques for regio- and stereospecific preparation of enolates
and enolate equivalents. If the reaction is carried out with a stoichiometrically formed
enolate in the absence of a proton source, the initial product is the enolate of the
adduct. The replacement of a bond by a bond ensures a favorable
H.
Among Michael acceptors that have been shown to react with ketone and
ester enolates under kinetic conditions are methyl -trimethylsilylvinyl ketone, 295
methyl -methylthioacrylate, 296 methyl methylthiovinyl sulfoxide, 297 and ethyl
-cyanoacrylate. 298 Each of these acceptors benefits from a second anion-stabilizing
substituent. The latter class of acceptors has been found to be capable of generating
contiguous quaternary carbon centers.
CN
CH 3 O Li + CO C H CHCO C H
–
C C + 2 2 5 2 2 5
2
CH 3 OCH 3 CN C CO CH 3
CH 3 CH 3
Ref. 298
Several examples of conjugate addition of carbanions carried out under aprotic
conditions are given in Scheme 2.24. The reactions are typically quenched by addition
of a proton source to neutralize the enolate. It is also possible to trap the adduct
by silylation or, as we will see in Section 2.6.2, to carry out a tandem alkylation.
Lithium enolates preformed by reaction with LDA in THF react with enones to give
1,4-diketones (Entries 1 and 2). Entries 3 and 4 involve addition of ester enolates to
enones. The reaction in Entry 3 gives the 1,2-addition product at −78 C but isomerizes
to the 1,4-product at 25 C. Esters of 1,5-dicarboxylic acids are obtained by addition
of ester enolates to , -unsaturated esters (Entry 5). Entries 6 to 8 show cases of
293
S. Tori, H. Tanaka, and Y. Kobayashi, J. Org. Chem., 42, 3473 (1977).
294 J. H. Clark, J. M. Miller, and K.-H. So, J. Chem. Soc., Perkin Trans. I, 941 (1978).
295
G. Stork and B. Ganem, J. Am. Chem. Soc., 95, 6152 (1973).
296
R. J. Cregge, J. L. Herrmann, and R. H. Schlessinger, Tetrahedron Lett., 2603 (1973).
297 J. L. Herrmann, G. R. Kieczykowski, R. F. Romanet, P. J. Wepple, and R. H. Schlessinger, Tetrahedron
Lett., 4711 (1973).
298
R. A. Holton, A. D. Williams, and R. M. Kennedy, J. Org. Chem., 51, 5480 (1986).