Page 215 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 215
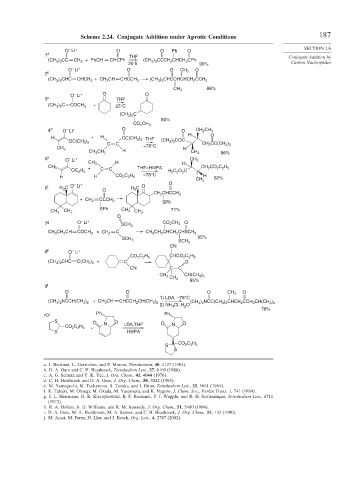
Scheme 2.24. Conjugate Addition under Aprotic Conditions 187
–
O Li + O O Ph O SECTION 2.6
1 a THF Conjugate Addition by
(CH 3 ) 3 CC CH 2 + PhCH CHCPh (CH 3 ) 3 CCCH 2 CHCH 2 CPh
20 h 90% Carbon Nucleophiles
–
O Li + O O CH 3 O
2 b
(CH 3 ) 2 CHC CHCH 3 + CH 3 CH CHCCH 3 (CH 3 ) 2 CHCCHCHCH 2 CCH 3
88%
CH 3
–
O Li + O O
3 c THF
(CH 3 ) 2 C COCH 3 + 25°C
(CH 3 ) 2 C
83%
CO 2 CH 3
–
4 d O Li + O O CH 2 CH 3
H O
H + H CC(CH 3 ) 3 THF (CH 3 ) 3 COC
OC(CH 3 ) 3
C C CH 2 CC(CH 3 ) 3
–78°C
CH 3 H H
CH 3 CH 2 CH 3 86%
5 e O Li + CH 3
–
CH 3 H H
+ C C
CH 3 THF–HMPA CH 2 CO 2 C 2 H 5
OC 2 H 5 H 5 C 2 O 2 C
–78°C
H H CO 2 C 2 H 5 H 82%
CH 3
O
+
–
6 f H C O Li O H 3 C O
3
CH 2 CHCCH 3
+ CH 2 CCCH 3
SPh
SPh 71%
CH 3 CH 3 CH 3 CH 3
O
–
7 g O Li + CO 2 CH 3 O
SCH 3
CH 3 CH 2 CH COCH 3 + CH 2 C CH 3 CH 2 CHCH 2 CHSCH 3
95%
SCH 3
SCH 3
CN
–
8 h O Li +
CO 2 C 2 H 5 CHCO 2 C 2 H 5
(CH 3 ) 2 CHC C(CH 3 ) 2 + C O
CN C C
CH 3 CH(CH 3 ) 2
CH 3
95%
9 i
O O O CH 3 O
1) LDA, –78°C
(CH 3 ) 2 NCCH(CH 3 ) 2 + CH 3 CH CHCCH 2 CH(CH 3 ) 2 (CH 3 ) 2 NCC(CH 3 ) 2 CHCH 2 CCH 2 CH(CH 3 ) 2
2) NH 4 Cl, H 2 O
78%
10 j Ph Ph
S O N O LDA,THF O N O
CO 2 C 2 H 5 +
S HMPA
S CO 2 C 2 H 5
S
a. J. Bertrand, L. Gorrichon, and P. Maroni, Tetrahedron, 40, 4127 (1984).
b. D. A. Oare and C. H. Heathcock, Tetrahedron Lett., 27, 6169 (1986).
c. A. G. Schultz and Y. K. Yee, J. Org. Chem., 41, 4044 (1976).
d. C. H. Heathcock and D. A. Oare, J. Org. Chem., 50, 3022 (1985).
e. M. Yamaguchi, M. Tsukamoto, S. Tanaka, and I. Hirao, Tetrahedron Lett., 25, 5661 (1984).
f. K. Takaki, M. Ohsugi, M. Okada, M. Yasumura, and K. Negoro, J. Chem. Soc., Perkin Trans. 1, 741 (1984).
g. J. L. Herrmann, G. R. Kieczykowski, R. F. Romanet, P. J. Wepplo, and R. H. Schlessinger, Tetrahedron Lett., 4711
(1973).
h. R. A. Holton, A. D. Williams, and R. M. Kennedy, J. Org. Chem., 51, 5480 (1986).
i. D. A. Oare, M. A. Henderson, M. A. Sanner, and C. H. Heathcock, J. Org. Chem., 55, 132 (1990).
j. M. Amat, M. Perez, N. Llor, and J. Bosch, Org. Lett., 4, 2787 (2002).