Page 218 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 218
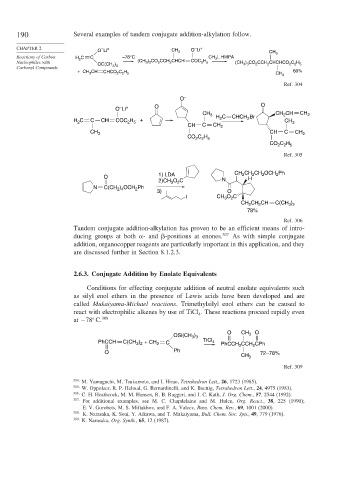
190 Several examples of tandem conjugate addition-alkylation follow.
CHAPTER 2 – + – +
O Li CH 3 O Li CH
Reactions of Carbon H C C –78°C CH I, HMPA 3
3
2
Nucleophiles with (CH ) CO CCH CHCH COC H (CH ) CO CCH CHCHCO C H
2 5
2
3 3
2
)
OC(CH 3 3 3 3 2 2 2 2 5
Carbonyl Compounds
+ CH CH C H 60%
3 CHCO 2 2 5 CH 3
Ref. 304
O –
–
O Li + O O
CH CH
CH 3 2 CH 2
H 2 C CHCH Br
2
H C C CH COC H + CH 3
2 5
2
CH C CH 2
CH 3 CH C CH 2
CO C H
2 2 5
C H
CO 2 2 5
Ref. 305
1) LDA CH CH CH OCH Ph
2
2
2
2
O
2)CH O C N H
3
2
N C(CH ) OCH Ph
2 4
2
3) O
I CH 3 O C
2
2
CH 2 CH CH C(CH )
3 2
78%
Ref. 306
Tandem conjugate addition-alkylation has proven to be an efficient means of intro-
ducing groups at both - and -positions at enones. 307 As with simple conjugate
addition, organocopper reagents are particularly important in this application, and they
are discussed further in Section 8.1.2.3.
2.6.3. Conjugate Addition by Enolate Equivalents
Conditions for effecting conjugate addition of neutral enolate equivalents such
as silyl enol ethers in the presence of Lewis acids have been developed and are
called Mukaiyama-Michael reactions. Trimethylsilyl enol ethers can be caused to
react with electrophilic alkenes by use of TiCl . These reactions proceed rapidly even
4
at −78 C. 308
O CH
OSi(CH ) 3 O
3 3
PhCCH C(CH ) + CH 2 C TiCl 4 PhCCH 2 CCH CPh
3 2
2
O Ph 72–78%
CH 3
Ref. 309
304
M. Yamaguchi, M. Tsukamoto, and I. Hirao, Tetrahedron Lett., 26, 1723 (1985).
305 W. Oppolzer, R. P. Heloud, G. Bernardinelli, and K. Baettig, Tetrahedron Lett., 24, 4975 (1983).
306 C. H. Heathcock, M. M. Hansen, R. B. Ruggeri, and J. C. Kath, J. Org. Chem., 57, 2544 (1992).
307
For additional examples, see M. C. Chapdelaine and M. Hulce, Org. React., 38, 225 (1990);
E. V. Gorobets, M. S. Miftakhov, and F. A. Valeev, Russ. Chem. Rev., 69, 1001 (2000).
308 K. Narasaka, K. Soai, Y. Aikawa, and T. Mukaiyama, Bull. Chem. Soc. Jpn., 49, 779 (1976).
309
K. Narasaka, Org. Synth., 65, 12 (1987).