Page 221 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 221
Fluoride ion can also induce reaction of silyl ketene acetals with electrophilic 193
alkenes. The fluoride source in these reactions is tris-(dimethylamino)sulfonium diflu-
orotrimethylsilicate (TASF). SECTION 2.6
Conjugate Addition by
Carbon Nucleophiles
OCH3 CHCO CH 3
2
O + CH 3 CH C F – O CH
OSi(CH ) 3
3 3
Ref. 318
Enamines also react with electrophilic alkenes to give conjugate addition products.
The addition reactions of enamines of cyclohexanones show a strong preference for
attack from the axial direction. 319 This is anticipated on stereoelectronic grounds
because the orbital of the enamine is the site of nucleophilicity.
O
C CHCPh
H 2
H
H H CH CPh
O H O CH 2 2
Ph 2
O
NR
H 2 H NR 2 H O
Scheme 2.25 shows some examples of additions of enolate equivalents. A range of
Lewis acid catalysts has been used in addition to TiCl and SnCl . Entry 1 shows uses
4
4
of a lanthanide catalyst. Entry 2 employs LiClO as the catalyst. The reaction in Entry
4
3 includes a chiral auxiliary that controls the stereoselectivity; the chiral auxiliary
is released by a cyclization using N-methylhydroxylamine. Entries 4 and 5 use the
triphenylmethyl cation as a catalyst and Entries 6 and 7 use trimethylsilyl triflate and
an enantioselective catalyst, respectively.
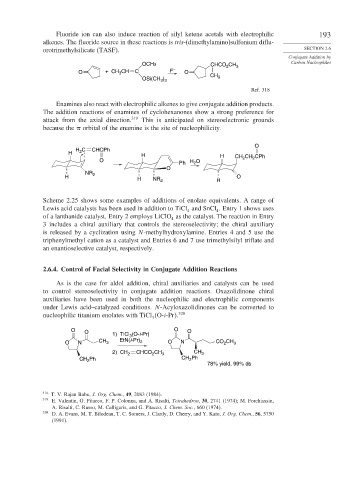
2.6.4. Control of Facial Selectivity in Conjugate Addition Reactions
As is the case for aldol addition, chiral auxiliaries and catalysts can be used
to control stereoselectivity in conjugate addition reactions. Oxazolidinone chiral
auxiliaries have been used in both the nucleophilic and electrophilic components
under Lewis acid–catalyzed conditions. N-Acyloxazolidinones can be converted to
nucleophilic titanium enolates with TiCl O-i-Pr . 320
3
O O 1) TiCl (O-i-Pr) O O
3
O N CH 3 EtN(i-Pr) 2 O N CO CH 3
2
2) CH 2 CHCO CH 3 CH 3
2
CH Ph CH 2 Ph
2
78% yield, 99% ds
318
T. V. Rajan Babu, J. Org. Chem., 49, 2083 (1984).
319 E. Valentin, G. Pitacco, F. P. Colonna, and A. Risalti, Tetrahedron, 30, 2741 (1974); M. Forchiassin,
A. Risalti, C. Russo, M. Calligaris, and G. Pitacco, J. Chem. Soc., 660 (1974).
320
D. A. Evans, M. T. Bilodeau, T. C. Somers, J. Clardy, D. Cherry, and Y. Kato, J. Org. Chem., 56, 5750
(1991).