Page 224 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 224
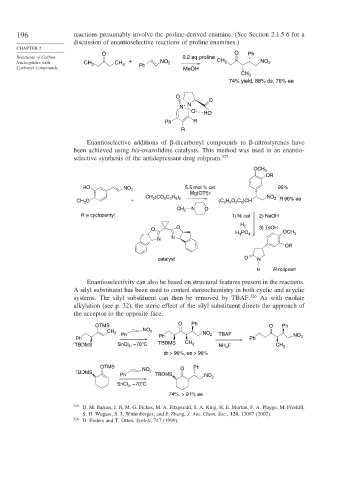
196 reactions presumably involve the proline-derived enamine. (See Section 2.1.5.6 for a
discussion of enantioselective reactions of proline enamines.)
CHAPTER 2
O O Ph
Reactions of Carbon 0.2 eq proline
Nucleophiles with CH 3 CH 3 + NO 2 CH 3 NO 2
Carbonyl Compounds Ph MeOH
CH 3
74% yield, 88% ds, 76% ee
O
O
N + N
O –
HO
Ph R
R
Enantioselective additions of -dicarbonyl compounds to -nitrostyrenes have
been achieved using bis-oxazolidine catalysts. This method was used in an enantio-
selective synthesis of the antidepressant drug rolipram. 325
OCH 3
OR
RO NO 2 5.5 mol % cat 95%
Mg(OTf)2
CH (CO C H ) NO 2 R 96% ee
O + 2 2 2 5 2 (C H O C )CH
CH 3 2 5 2 2
N
CH 3 O
R = cyclopentyl 1) Ni cat 2) NaOH
O O H 2 3) TsOH
H PO 4 OCH 3
3
N N
OR
catalyst O N
H R-rolipram
Enantioselectivity can also be based on structural features present in the reactants.
A silyl substituent has been used to control stereochemistry in both cyclic and acyclic
systems. The silyl substituent can then be removed by TBAF. 326 As with enolate
alkylation (see p. 32), the steric effect of the silyl substituent directs the approach of
the acceptor to the opposite face.
OTMS O Ph O Ph
CH 3 NO 2 NO 2 TBAF
Ph Ph Ph Ph NO 2
TBDMS SnCl 4 , –70°C TBDMS CH 3 NH F CH 3
4
dr > 96%, ee > 96%
OTMS O Ph
NO 2
TBDMS
Ph TBDMS NO 2
, –70°C
SnCl 4
74%, > 91% ee
325 D. M. Barnes, J. Ji, M. G. Fickes, M. A. Fitzgerald, S. A. King, H. E. Morton, F. A. Plagge, M. Preskill,
S. H. Wagaw, S. J. Wittenberger, and J. Zhang, J. Am. Chem. Soc., 124, 13097 (2002).
326
D. Enders and T. Otten, Synlett, 747 (1999).