Page 225 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 225
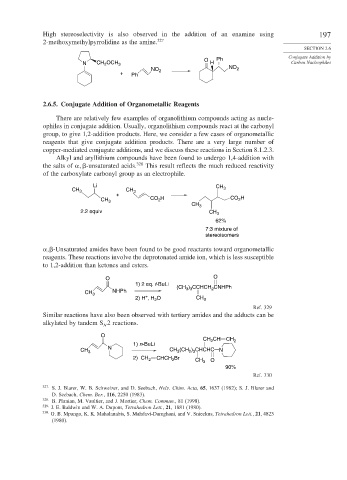
High stereoselectivity is also observed in the addition of an enamine using 197
2-methoxymethylpyrrolidine as the amine. 327
SECTION 2.6
O Ph Conjugate Addition by
N CH OCH 3 H Carbon Nucleophiles
2
NO 2
NO 2
+ Ph
2.6.5. Conjugate Addition of Organometallic Reagents
There are relatively few examples of organolithium compounds acting as nucle-
ophiles in conjugate addition. Usually, organolithium compounds react at the carbonyl
group, to give 1,2-addition products. Here, we consider a few cases of organometallic
reagents that give conjugate addition products. There are a very large number of
copper-mediated conjugate additions, and we discuss these reactions in Section 8.1.2.3.
Alkyl and aryllithium compounds have been found to undergo 1,4-addition with
the salts of -unsaturated acids. 328 This result reflects the much reduced reactivity
of the carboxylate carbonyl group as an electrophile.
Li CH
CH 3 CH 3 3
+
CH 3 CO 2 H CO H
2
CH 3
2.2 equiv CH 3
62%
7:3 mixture of
stereoisomers
-Unsaturated amides have been found to be good reactants toward organometallic
reagents. These reactions involve the deprotonated amide ion, which is less susceptible
to 1,2-addition than ketones and esters.
O O
1) 2 eq. t-BuLi
) CCHCH CNHPh
(CH 3 3 2
CH 3 NHPh
+
2) H , H O CH 3
2
Ref. 329
Similar reactions have also been observed with tertiary amides and the adducts can be
alkylated by tandem S 2 reactions.
N
O
CH CH CH 2
2
1) n-BuLi
N
CH 3 CH (CH ) CHCHC N
2 3
3
2) CH 2 CHCH Br CH 3 O
2
90%
Ref. 330
327 S. J. Blarer, W. B. Schweizer, and D. Seebach, Helv. Chim. Acta, 65, 1637 (1982); S. J. Blarer and
D. Seebach, Chem. Ber., 116, 2250 (1983).
328
B. Plunian, M. Vaultier, and J. Mortier, Chem. Commun., 81 (1998).
329 J. E. Baldwin and W. A. Dupont, Tetrahedron Lett., 21, 1881 (1980).
330
G. B. Mpango, K. K. Mahalanabis, S. Mahdavi-Damghani, and V. Snieckus, Tetrahedron Lett., 21, 4823
(1980).