Page 222 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 222
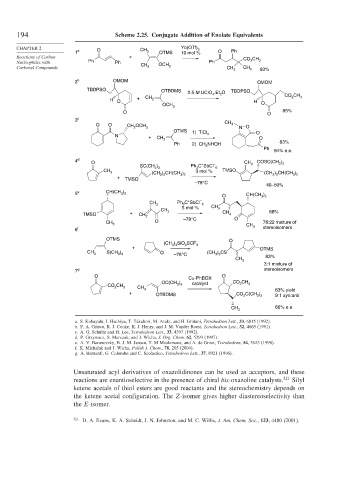
194 Scheme 2.25. Conjugate Addition of Enolate Equivalents
CHAPTER 2 O Yb(OTf) 3
1 a CH 3 OTMS 10 mol % O Ph
Reactions of Carbon + CO CH
Nucleophiles with Ph Ph CH OCH Ph 2 3
Carbonyl Compounds 3 3 CH 3 CH 3 93%
2 b OMOM OMOM
TBDPSO OTBDMS 2.5 M LiClO .Et O TBDPSO
4
2
H O + CH 2 H CO 2 CH 3
OCH O
3
85%
O O
3 c
O O CH 2 OCH 3 CH 3 N O
OTMS 1) TiCl O
N + CH 2 4 O
Ph 2) CH NHOH 83%
3
Ph
94% e.e.
4 d O COSC(CH )
+
SC(CH ) Ph C SbCl – CH 3 3 3
3 3 3 6
3 mol % TMSO
CH 3 ) CH(CH ) (CH ) CH(CH )
(CH 2 3 3 2 2 3 3 2
+
TMSO
–78°C
40–50%
3 2
5 e CH(CH ) CH(CH )
O 3 2
+
CH Ph C SbCl –
3 3 6 CH
5 mol % 3
CH 3 68%
TMSO + CH 2 CH 3 O
CH 3 O –78°C CH 78:22 mixture of
6 f 3 stereoisomers
OTMS O
(CH ) SiO SCF 3
3 3
3
+ OTMS
S(CH ) O (CH ) CS
CH 3 3 3 –78°C 3 3
83%
CH 3
3:1 mixture of
7 g stereoisomers
O Cu-PhBOX O
OC(CH ) catalyst CO CH
CH 3 3 2 3
CO 2 CH
3
3 63% yield
+ OTBDMS CO C(CH ) 9:1 syn:anti
3 3
2
CH 3 66% e.e.
a. S. Kobayahi, I. Hachiya, T. Takahori, M. Araki, and H. Ishitani, Tetrahedron Lett., 33, 6815 (1992).
b. P. A. Grieco, R. J. Cooke, K. J. Henry, and J. M. Vander Roest, Tetrahedron Lett., 32, 4665 (1991).
c. A. G. Schultz and H. Lee, Tetrahedron Lett., 33, 4397 (1992).
d. P. Grzywacz, S. Marczak, and J. Wicha, J. Org. Chem. 62, 5293 (1997).
e. A. V. Baranovsky, B. J. M. Jansen, T. M Meulemans, and A. de Groot, Tetrahedron, 54, 5623 (1998).
f. K. Michalak and J. Wicha, Polish J. Chem., 78, 205 (2004).
g. A. Bernardi, G. Colombo and C. Scolastico, Tetrahedron Lett., 37, 8921 (1996).
Unsaturated acyl derivatives of oxazolidinones can be used as acceptors, and these
reactions are enantioselective in the presence of chiral bis-oxazoline catalysts. 321 Silyl
ketene acetals of thiol esters are good reactants and the stereochemistry depends on
the ketene acetal configuration. The Z-isomer gives higher diastereoselectivity than
the E-isomer.
321
D. A. Evans, K. A. Scheidt, J. N. Johnston, and M. C. Willis, J. Am. Chem. Soc., 123, 4480 (2001).