Page 219 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 219
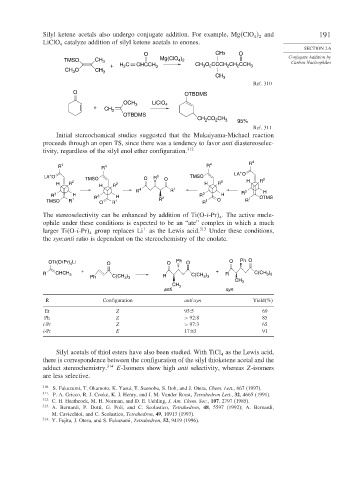
Silyl ketene acetals also undergo conjugate addition. For example, Mg ClO and 191
4 2
LiClO catalyze addition of silyl ketene acetals to enones.
4
SECTION 2.6
O CH3 O Conjugate Addition by
)
TMSO CH 3 Mg(ClO 4 2 Carbon Nucleophiles
+ H C CHCCH 3 CH 3 O CCCH CH CCH 3
2
2
2
2
CH O CH 3
3
CH 3
Ref. 310
O OTBDMS
OCH 3 LiClO 4
+ CH 2
OTBDMS
CH CO CH 3 95%
2
2
Ref. 311
Initial stereochemical studies suggested that the Mukaiyama-Michael reaction
proceeds through an open TS, since there was a tendency to favor anti diastereoselec-
tivity, regardless of the silyl enol ether configuration. 312
R 4 R 4 R 4 R 4
+
LA O
+
LA O 3 TMSO
TMSO O R O 2
H R 2 H R 2 H R 2 H R
R 4 R 1 R 3 H
R 3 H R 3 H R 3 H OTMS
TMSO R 1 O R 1 R 2 R 1 O R 1
The stereoselectivity can be enhanced by addition of Ti O-i-Pr . The active nucle-
4
ophile under these conditions is expected to be an “ate” complex in which a much
+
larger Ti O-i-Pr group replaces Li as the Lewis acid. 313 Under these conditions,
4
the syn:anti ratio is dependent on the stereochemistry of the enolate.
OTi(Oi Pr) Li O O Ph O O Ph O
4
+ +
)
R CHCH 3 C(CH ) R C(CH 3 3
)
Ph C(CH 3 3 R 3 3
CH 3
CH 3
anti syn
R Configuration anti:syn Yield(%)
Et Z 95:5 69
Ph Z > 92 8 85
i-Pr Z > 97 3 65
i-Pr E 17:83 91
Silyl acetals of thiol esters have also been studied. With TiCl as the Lewis acid,
4
there is correspondence between the configuration of the silyl thioketene acetal and the
adduct stereochemistry. 314 E-Isomers show high anti selectivity, whereas Z-isomers
are less selective.
310 S. Fukuzumi, T. Okamoto, K. Yasui, T. Suenobu, S. Itoh, and J. Otera, Chem. Lett., 667 (1997).
311 P. A. Grieco, R. J. Cooke, K. J. Henry, and J. M. Vander Roest, Tetrahedron Lett., 32, 4665 (1991).
312
C. H. Heathcock, M. H. Norman, and D. E. Uehling, J. Am. Chem. Soc., 107, 2797 (1985).
313 A. Bernardi, P. Dotti, G. Poli, and C. Scolastico, Tetrahedron, 48, 5597 (1992); A. Bernardi,
M. Cavicchioi, and C. Scolastico, Tetrahedron, 49, 10913 (1993).
314
Y. Fujita, J. Otera, and S. Fukuzumi, Tetrahedron, 52, 9419 (1996).