Page 223 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 223
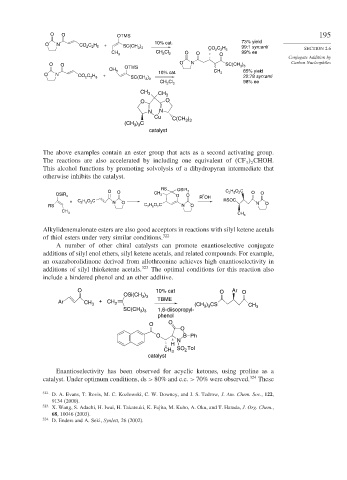
O O OTMS 195
O N CO C H + SC(CH ) 10% cat. 73% yield
5
2
2
3 3
CO 2 C H 99:1 syn:anti SECTION 2.6
CH Cl O 2 5 99% ee
CH 3 2 2 O O
Conjugate Addition by
O O O N SC(CH 3 ) 3 Carbon Nucleophiles
OTMS
CH 3 65% yield
O N CO C H 5 + 10% cat. CH 3 22:78 syn:anti
2
2
SC(CH 3 ) 3
CH Cl 2 98% ee
2
CH 3
CH 3
O O
N N
Cu )
C(CH 3 3
(CH ) C
3 3
catalyst
The above examples contain an ester group that acts as a second activating group.
The reactions are also accelerated by including one equivalent of CF CHOH.
3 2
This alcohol functions by promoting solvolysis of a dihydropyran intermediate that
otherwise inhibits the catalyst.
RS
O O OSiR 3 C 2 H O C O
OSiR 3 CH 3 O O R OH 5 2 O
F
+ C H O C N O RSOC N
5
2
2
RS C 2 H O C N O O
2
5
CH
CH 3
3
Alkylidenemalonate esters are also good acceptors in reactions with silyl ketene acetals
of thiol esters under very similar conditions. 322
A number of other chiral catalysts can promote enantioselective conjugate
additions of silyl enol ethers, silyl ketene acetals, and related compounds. For example,
an oxazaborolidinone derived from allothreonine achieves high enantioselectivity in
additions of silyl thioketene acetals. 323 The optimal conditions for this reaction also
include a hindered phenol and an ether additive.
O 10% cat O Ar O
OSi(CH )
3 3
Ar CH 3 + CH 2 TBME (CH ) CS
SC(CH ) 1,6-diisopropyl- 3 3 CH 3
3 3
phenol
O O
O
O B Ph
N
H
CH 3 SO 2 Tol
catalyst
Enantioselectivity has been observed for acyclic ketones, using proline as a
catalyst. Under optimum conditions, ds > 80% and e.e. > 70% were observed. 324 These
322
D. A. Evans, T. Rovis, M. C. Kozlowski, C. W. Downey, and J. S. Tedrow, J. Am. Chem. Soc., 122,
9134 (2000).
323 X. Wang, S. Adachi, H. Iwai, H. Takatsuki, K. Fujita, M. Kubo, A. Oku, and T. Harada, J. Org. Chem.,
68, 10046 (2003).
324
D. Enders and A. Seki, Synlett, 26 (2002).