Page 213 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 213
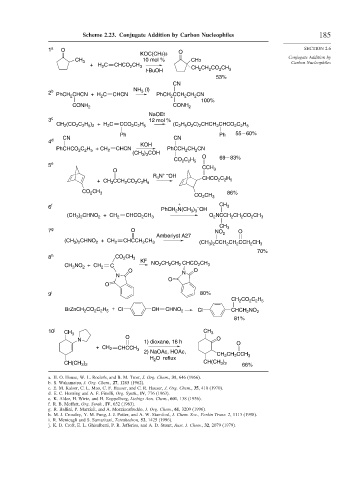
Scheme 2.23. Conjugate Addition by Carbon Nucleophiles 185
1 a O SECTION 2.6
KOC(CH3)3 O
CH 3 10 mol % CH3 Conjugate Addition by
Carbon Nucleophiles
+ H C CHCO CH 3 CH CH CO CH
2
2
t-BuOH 2 2 2 3
53%
CN
3
2 b PhCH CHCN + H C CHCN NH (l) PhCH CCH CH CN
2
2
2
2
2
100%
CONH 2 CONH 2
NaOEt
3 c 12 mol %
CH (CO 2 C 2 H 5 ) 2 + H 2 C CCO 2 C 2 H 5 (C H 5 O 2 C) 2 CHCH 2 CHCO 2 C 2 H 5
2
2
Ph Ph 55 – 60%
CN CN
4 d
KOH
C H + CH CHCN
PhCHCO 2 2 5 2 PhCCH CH CN
2
2
) COH
(CH 3 3 O 69 – 83%
C H
CO 2 2 5
5 e CCH
O 3
R N + – OH
4
+ CH CCH CO C H CHCO C H
2 2 5
2 2 5
3
2
CO CH 3 CO CH 3 86%
2
2
6 f + – CH 3
PhCH N(CH ) OH
2
3 3
(CH ) CHNO + CH 2 CHCO CH 3 O NCCH CH CO CH 3
2
2
2
2
2
2
3 2
CH 3
7 g O NO O
Amberlyst A27 2
(CH ) CHNO + CH 2 CHCCH CH 3 (CH ) CCH CH CCH CH 3
2
2
3 2
3 2
2
2
2
70%
8 h CO CH 3
2
KF CH CH CHCO CH
CH NO + CH 2 C NO 2 2 2 2 3
2
3
O N O
N
O
O
9 i 80%
CH CO C H
2
2 2 5
BrZnCH CO C H + Cl CH CHNO 2 Cl CHCH NO 2
2 2 5
2
2
81%
10 j CH 3 CH 3
O
N 1) dioxane, 16 h O O
+ CH 2 CHCCH 3
2) NaOAc, HOAc, CH CH CCH
H O reflux 2 2 3
2
CH(CH ) CH(CH ) 66%
3 2
3 2
a. H. O. House, W. L. Roelofs, and B. M. Trost, J. Org. Chem., 31, 646 (1966).
b. S. Wakamatsu, J. Org. Chem., 27, 1285 (1962).
c. E. M. Kaiser, C. L. Mao, C. F. Hauser, and C. R. Hauser, J. Org. Chem., 35, 410 (1970).
d. E. C. Horning and A. F. Finelli, Org. Synth., IV, 776 (1963).
e. K. Alder, H. Wirtz, and H. Koppelberg, Liebigs Ann. Chem., 601, 138 (1956).
f. R. B. Moffett, Org. Synth., IV, 652 (1963).
g. R. Ballini, P. Marziali, and A. Mozziacafreddo, J. Org. Chem., 61, 3209 (1996).
h. M. J. Crossley, Y. M. Fung, J. J. Potter, and A. W. Stamford, J. Chem. Soc., Perkin Trans. 2, 1113 (1998).
i. R. Menicagli and S. Samaritani, Tetrahedron, 52, 1425 (1996).
j. K. D. Croft, E. L. Ghisalberti, P. R. Jefferies, and A. D. Stuart, Aust. J. Chem., 32, 2079 (1979).