Page 242 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 242
214 OCH3 OCH3
from
CHAPTER 2
HO CCH CH
Reactions of Carbon 2
Nucleophiles with
Carbonyl Compounds N
R enantiomer of the antidepressant drug Rolipram
H
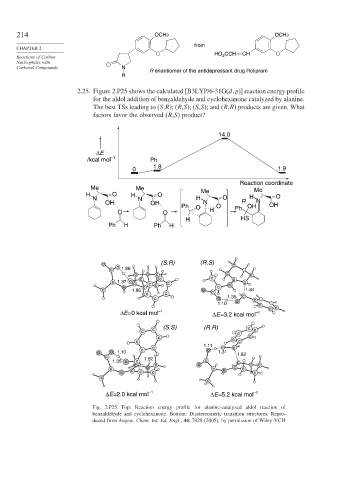
2.25. Figure 2.P25 shows the calculated [B3LYP/6-31G(d p)] reaction energy profile
for the aldol addition of benzaldehyde and cyclohexanone catalyzed by alanine.
The best TSs leading to (S,R); (R,S); (S,S); and (R,R) products are given. What
factors favor the observed (R,S) product?
14.0
ΔE
/kcal mol –1 Ph
1.8
0 1.9
Reaction coordinate
Me Me Me
H O H O Me H O
N N H O N
OH OH N R
Ph O O Ph OH OH
O O H
H HS
Ph H Ph H
o (S.R) (R.S) c
o
c 1.86
o o c c c
c c c c
c 1.37 c c c c c c
N c c N
c 1.86 o c 1.84
c c c
c o 1.35 c
1.10 o c
c c
ΔE= 0 kcal mol –1 ΔE=3.2 kcal mol –1
c (S.S) (R.R) c c
c
c
c c
c c c
1.11
c c
c o
o o 1.10 1.31 1.82
c 1.82
1.35 o c c c
c c o c
c c c c N c c
N c c c
c
c
ΔE=2.0 kcal mol –1 ΔE=5.2 kcal mol –1
Fig. 2.P25. Top: Reaction energy profile for alanine-catalyzed aldol reaction of
benzaldehyde and cyclohexanone. Bottom: Diastereomeric transition structures. Repro-
duced from Angew. Chem. Int. Ed. Engl., 44, 7028 (2005), by permission of Wiley-VCH