Page 237 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 237
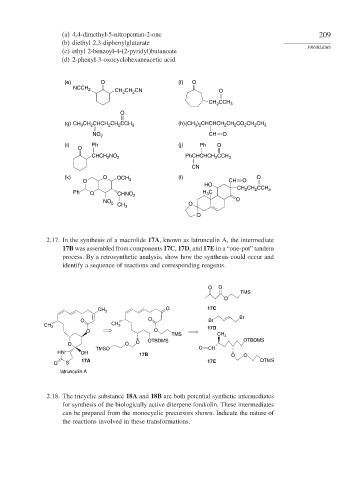
(a) 4,4-dimethyl-5-nitropentan-2-one 209
(b) diethyl 2,3-diphenylglutarate
PROBLEMS
(c) ethyl 2-benzoyl-4-(2-pyridyl)butanoate
(d) 2-phenyl-3-oxocyclohexaneacetic acid
(e) O (f) O
NCCH 2
CH CH CN O
2
2
CH CCH 3
2
O
(g) CH CH CHCH CH CCH 3 (h)(CH ) CHCHCH CH CO CH CH 3
2
2
3
2
2
2
3 2
2
2
NO 2 CH O
(i) Ph (j) Ph O
O
CHCH NO 2 PhCHCHCH 2 CCH 3
2
CN
(k) O OCH (l) O
O 3 CH O
HO
CH 2 CH CCH 3
2
Ph O CHNO 2 H C
3
NO 2 CH 3 O O
O
2.17. In the synthesis of a macrolide 17A, known as latrunculin A, the intermediate
17B was assembled from components 17C, 17D, and 17E in a “one-pot” tandem
process. By a retrosynthetic analysis, show how the synthesis could occur and
identify a sequence of reactions and corresponding reagents.
O O
TMS
O
CH 3 O 17C
Br
O O Br
CH
CH 3 3 17D
O O
TMS CH 3
O O O OTBDMS OTBDMS
TMSO O CH
HN OH 17B O O
17A 17E OTMS
O S
latrunculin A
2.18. The tricyclic substance 18A and 18B are both potential synthetic intermediates
for synthesis of the biologically active diterpene forskolin. These intermediates
can be prepared from the monocyclic precursors shown. Indicate the nature of
the reactions involved in these transformations.