Page 235 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 235
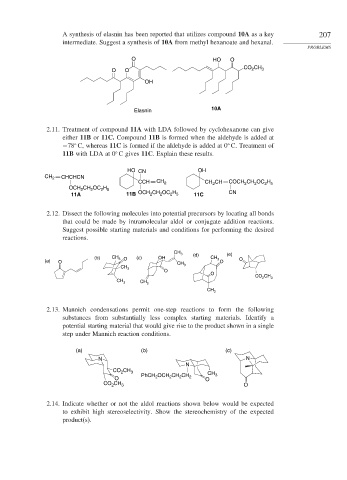
A synthesis of elasnin has been reported that utilizes compound 10A as a key 207
intermediate. Suggest a synthesis of 10A from methyl hexanoate and hexanal.
PROBLEMS
O HO O
O O CO 2 CH 3
OH
10A
Elasnin
2.11. Treatment of compound 11A with LDA followed by cyclohexanone can give
either 11B or 11C. Compound 11B is formed when the aldehyde is added at
−78 C, whereas 11C is formed if the aldehyde is added at 0 C. Treatment of
11B with LDA at 0 C gives 11C. Explain these results.
HO CN OH
CH 2 CHCHCN
CCH CH 2 CH CH COCH CH OC H
2
2
2
2 5
OCH CH OC H
2 5
2
2
2
2 5
2
11A 11B OCH CH OC H 11C CN
2.12. Dissect the following molecules into potential precursors by locating all bonds
that could be made by intramolecular aldol or conjugate addition reactions.
Suggest possible starting materials and conditions for performing the desired
reactions.
CH 3 (e)
(b) CH 3 O (c) OH (d) CH O
(a) O CH 3 O
CH 3
3
O
O
CO CH
CH CH 2 3
3 2
CH 3
2.13. Mannich condensations permit one-step reactions to form the following
substances from substantially less complex starting materials. Identify a
potential starting material that would give rise to the product shown in a single
step under Mannich reaction conditions.
(a) (b) (c)
N N
N
CO CH 3 CH
2
2
2
2
O PhCH OCH CH CH 2 O 3
CO 2 CH 3 O
2.14. Indicate whether or not the aldol reactions shown below would be expected
to exhibit high stereoselectivity. Show the stereochemistry of the expected
product(s).