Page 233 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 233
b. Reaction of the epoxide of E-4-octene (trans-2,3-dipropyloxirane) with 205
potassium trimethylsilanide gives Z-4-octene as the only alkene product in
PROBLEMS
93% yield. Suggest a reasonable mechanism for this reaction.
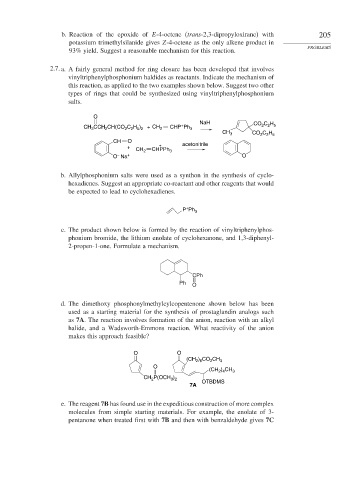
2.7. a. A fairly general method for ring closure has been developed that involves
vinyltriphenylphosphonium haldides as reactants. Indicate the mechanism of
this reaction, as applied to the two examples shown below. Suggest two other
types of rings that could be synthesized using vinyltriphenylphosphonium
salts.
O
NaH CO C H
+
CH CCH CH(CO C H ) + CH 2 CHP Ph 3 2 2 5
2
2 2 5 2
3
CH 3 CO C H
2 2 5
CH O acetonitrile
+
+ CH 2 CHPPh 3
–
O Na + O
b. Allylphosphonium salts were used as a synthon in the synthesis of cyclo-
hexadienes. Suggest an appropriate co-reactant and other reagents that would
be expected to lead to cyclohexadienes.
+
P Ph 3
c. The product shown below is formed by the reaction of vinyltriphenylphos-
phonium bromide, the lithium enolate of cyclohexanone, and 1,3-diphenyl-
2-propen-1-one. Formulate a mechanism.
CPh
Ph O
d. The dimethoxy phosphonylmethylcylcopentenone shown below has been
used as a starting material for the synthesis of prostaglandin analogs such
as 7A. The reaction involves formation of the anion, reaction with an alkyl
halide, and a Wadsworth-Emmons reaction. What reactivity of the anion
makes this approach feasible?
O O
(CH ) CO CH 3
2 6
2
O
) CH
(CH 2 4 3
CH 2 P(OCH ) OTBDMS
3 2
7A
e. The reagent 7B has found use in the expeditious construction of more complex
molecules from simple starting materials. For example, the enolate of 3-
pentanone when treated first with 7B and then with benzaldehyde gives 7C