Page 238 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 238
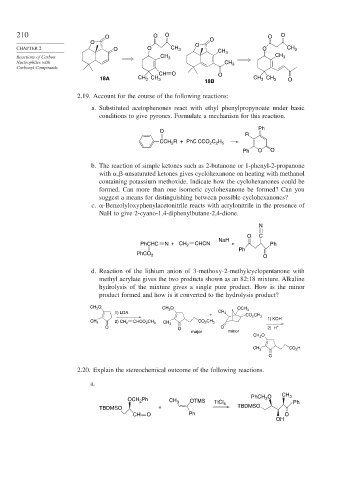
210 O O O O O
O O O
CHAPTER 2 O O CH 3 CH O CH 3
Reactions of Carbon CH 3 3 CH 3
Nucleophiles with CH 3
Carbonyl Compounds
CH O O
18A CH 3 CH 3 CH CH 3 O
3
18B
2.19. Account for the course of the following reactions:
a. Substituted acetophenones react with ethyl phenylpropynoate under basic
conditions to give pyrones. Formulate a mechanism for this reaction.
Ph
O
R
2 2 5
CCH 2 R + PhC CCO C H
Ph O O
b. The reaction of simple ketones such as 2-butanone or 1-phenyl-2-propanone
with -unsaturated ketones gives cyclohexanone on heating with methanol
containing potassium methoxide. Indicate how the cyclohexanones could be
formed. Can more than one isomeric cyclohexanone be formed? Can you
suggest a means for distinguishing between possible cyclohexanones?
c. -Benzolyloxyphenylacetonitrile reacts with acrylonitrile in the presence of
NaH to give 2-cyano-1,4-diphenylbutane-2,4-dione.
N
O C
NaH
PhCHC N + CH 2 CHCN Ph
Ph
PhCO 2 O
d. Reaction of the lithium anion of 3-methoxy-2-methylcyclopentanone with
methyl acrylate gives the two products shown as an 82:18 mixture. Alkaline
hydrolysis of the mixture gives a single pure product. How is the minor
product formed and how is it converted to the hydrolysis product?
CH 3 O CH 3 O OCH 3
1) LDA CH 3
+ CO 2 CH 3
1) KOH
CH 3 2) CH 2 CHCO 2 CH 3 CO 2 CH 3
CH 3
O O O 2) H +
major minor
CH 3 O
CO 2 H
CH 3
O
2.20. Explain the stereochemical outcome of the following reactions.
a.
PhCH 2 O CH 3
OCH Ph CH 3 OTMS TiCl 4 Ph
2
TBDMSO + TBDMSO
CH O Ph O
OH