Page 236 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 236
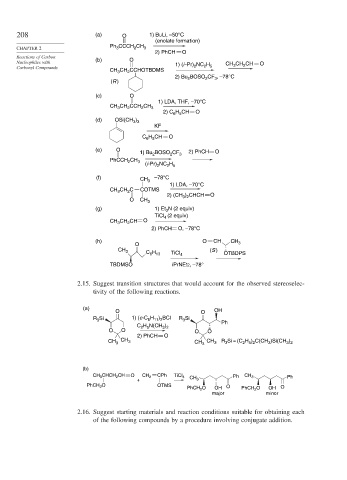
208 (a) O 1) BuLi, –50°C
(enolate formation)
CHAPTER 2 Ph 3 CCCH 2 CH 3
2) PhCH O
Reactions of Carbon (b) O
Nucleophiles with 1) (i -Pr) NC H CH CH CH O
Carbonyl Compounds 2 2 5 3 2
CH CH CCHOTBDMS
2
3
2) Bu BOSO 2 CF 3 , –78°C
2
(R )
(c) O
1) LDA, THF, –70°C
CH CH CCH CH 3
2
2
3
2) C 6 H 5 CH O
(d) OSi(CH )
3 3
KF
C H CH O
6 5
(e) O
1) Bu BOSO 2 2 CF 3 3 2) PhCH O
BOSO CF
2
1) Bu 2
PhCCH CH 3
2
(i-Pr) NC H
2
2 5
(f) CH 3 –78°C
1) LDA, –70°C
CH CH C COTMS
2
3
2) (CH ) CHCH O
3 2
O CH 3
(g) 1) Et 3 N (2 equiv)
TiCl (2 equiv)
4
CH CH CH O
2
3
2) PhCH O, –78°C
(h) O CH CH 3
O
CH 3 C H TiCl 4 (S ) OTBDPS
6 13
TBDMSO i PrNEt2, –78°
2.15. Suggest transition structures that would account for the observed stereoselec-
tivity of the following reactions.
(a)
O O OH
R Si 1) (c-C H ) BCl R Si
6 11 2
3
3
C H N(CH ) Ph
3 2
2 5
O O O O
2) PhCH O
CH 3 CH 3 CH CH 3 R Si = (C H ) C(CH )Si(CH )
2 5 2
3
3 2
3
3
(b)
CH 3 CHCH 2 CH O CH 2 CPh TiCl 4 Ph CH 3 Ph
+ CH 3
PhCH 2 O OTMS
PhCH 2 O OH O PhCH 2 O OH O
major minor
2.16. Suggest starting materials and reaction conditions suitable for obtaining each
of the following compounds by a procedure involving conjugate addition.