Page 232 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 232
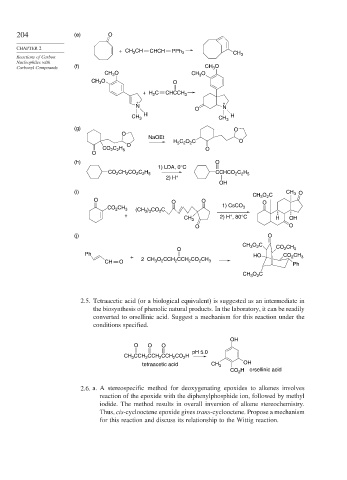
204 (e) O
CHAPTER 2
+ CH 3 CH CHCH PPh 3 CH
Reactions of Carbon 3
Nucleophiles with
Carbonyl Compounds (f) CH 3 O
CH O CH O
3
3
CH O O
3
+ H C CHCCH 3
2
+ +
N N
O
H
CH 3 CH 3 H
(g) O
O
NaOEt
H C O C O
2
5 2
O
CO C H O
2 2 5
O
(h) O
1) LDA, 0°C
CO 2 CH 2 CO 2 C 2 H 5 CCHCO C H
2 2 5
2) H +
OH
(i) CH 3 O
CH 3 O C
2
O O
O O
CO CH 3 (CH ) CO C 1) CsCO 3
2
3 3
2
+ +
CH 3 2) H , 80°C H OH
O O
(j) O
CH O C CO CH
3
2
O 2 3
Ph HO
+ 2 CH O CCH CCH CO CH CO 2 CH 3
CH O 3 2 2 2 2 3
Ph
CH O C
3
2
2.5. Tetraacetic acid (or a biological equivalent) is suggested as an intermediate in
the biosynthesis of phenolic natural products. In the laboratory, it can be readily
converted to orsellinic acid. Suggest a mechanism for this reaction under the
conditions specified.
OH
O O O
pH 5.0
CH CCH CCH CCH CO H
2
3
2
2
2
tetraacetic acid CH 3 OH
CO H orsellinic acid
2
2.6. a. A stereospecific method for deoxygenating epoxides to alkenes involves
reaction of the epoxide with the diphenylphosphide ion, followed by methyl
iodide. The method results in overall inversion of alkene stereochemistry.
Thus, cis-cyclooctene epoxide gives trans-cyclooctene. Propose a mechanism
for this reaction and discuss its relationship to the Wittig reaction.