Page 239 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 239
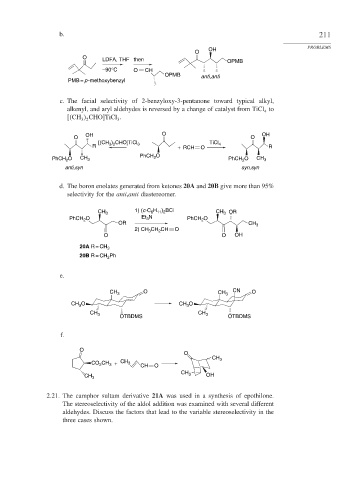
b. 211
PROBLEMS
OH
O
O LDFA, THF then OPMB
–90°C O CH
OPMB anti,anti
PMB = p -methoxybenzyl
c. The facial selectivity of 2-benzyloxy-3-pentanone toward typical alkyl,
alkenyl, and aryl aldehydes is reversed by a change of catalyst from TiCl to
4
CH CHO TiCl .
3
3 2
OH O OH
O O
[(CH ) CHO]TiCl TiCl
R 3 2 3 + RCH O 4 R
PhCH O
PhCH O CH 3 2 PhCH O CH 3
2
2
anti,syn syn,syn
d. The boron enolates generated from ketones 20A and 20B give more than 95%
selectivity for the anti,anti diastereomer.
H ) BCl
CH 3 1) (c-C 6 11 2 CH 3 OR
PhCH O Et 3 N PhCH O
2
2
OR CH 3
2) CH CH CH O
2
3
O O OH
20A R = CH 3
20B R = CH 2 Ph
e.
CH 3 O CH 3 CN O
CH O CH O
3
3
CH 3 OTBDMS CH 3 OTBDMS
f.
O
O
CH 3
CH CH 3
CO 2 3 + CH O
CH
CH 3 3 OH
2.21. The camphor sultam derivative 21A was used in a synthesis of epothilone.
The stereoselectivity of the aldol addition was examined with several different
aldehydes. Discuss the factors that lead to the variable stereoselectivity in the
three cases shown.