Page 339 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 339
E + E + 311
(C) n E E
(C) n (C) SECTION 4.2
(C) n n
Nu Nu: Nu Electrophilic Cyclization
Nu:
exo-trig cyclization endo-trig cyclization
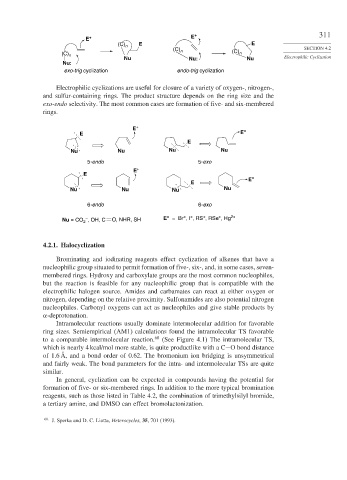
Electrophilic cyclizations are useful for closure of a variety of oxygen-, nitrogen-,
and sulfur-containing rings. The product structure depends on the ring size and the
exo-endo selectivity. The most common cases are formation of five- and six-membered
rings.
E +
E E +
E
Nu Nu Nu Nu
5-endo 5-exo
E +
E
E +
E
Nu Nu Nu Nu
6-endo 6-exo
+
+
+
+
+
–
Nu = CO , OH, C O, NHR, SH E = Br , I , RS , RSe , Hg 2+
2
4.2.1. Halocyclization
Brominating and iodinating reagents effect cyclization of alkenes that have a
nucleophilic group situated to permit formation of five-, six-, and, in some cases, seven-
membered rings. Hydroxy and carboxylate groups are the most common nucleophiles,
but the reaction is feasible for any nucleophilic group that is compatible with the
electrophilic halogen source. Amides and carbamates can react at either oxygen or
nitrogen, depending on the relative proximity. Sulfonamides are also potential nitrogen
nucleophiles. Carbonyl oxygens can act as nucleophiles and give stable products by
-deprotonation.
Intramolecular reactions usually dominate intermolecular addition for favorable
ring sizes. Semiempirical (AM1) calculations found the intramolecular TS favorable
to a comparable intermolecular reaction. 68 (See Figure 4.1) The intramolecular TS,
which is nearly 4 kcal/mol more stable, is quite productlike with a C−O bond distance
of 1.6 Å, and a bond order of 0.62. The bromonium ion bridging is unsymmetrical
and fairly weak. The bond parameters for the intra- and intermolecular TSs are quite
similar.
In general, cyclization can be expected in compounds having the potential for
formation of five- or six-membered rings. In addition to the more typical bromination
reagents, such as those listed in Table 4.2, the combination of trimethylsilyl bromide,
a tertiary amine, and DMSO can effect bromolactonization.
68
J. Sperka and D. C. Liotta, Heterocycles, 35, 701 (1993).