Page 343 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 343
Analogous cyclization reactions are induced by brominating reagents but they 315
83
tend to be less selective than the iodocyclizations. The bromonium ion intermediates
are much more reactive and less selective. SECTION 4.2
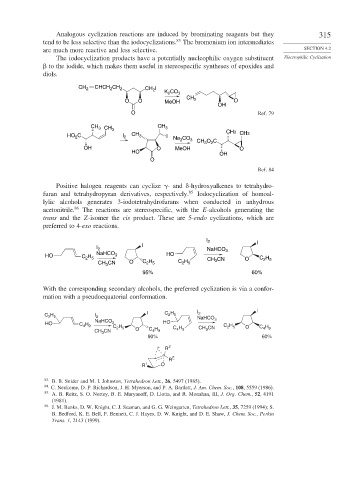
The iodocyclization products have a potentially nucleophilic oxygen substituent Electrophilic Cyclization
to the iodide, which makes them useful in stereospecific syntheses of epoxides and
diols.
CH 2 CHCH CH 2 CH I
2
2
K 2 CO 3
O O MeOH CH 2 O
OH
O Ref. 79
CH 3 CH 3 CH 3
HO C I 2 CH 3 I Na CO 3 CH O C CH3 CH3
2
2
OH O MeOH 3 2 O
HO
OH
O
Ref. 84
Positive halogen reagents can cyclize
- and -hydroxyalkenes to tetrahydro-
furan and tetrahydropyran derivatives, respectively. 85 Iodocyclization of homoal-
lylic alcohols generates 3-iodotetrahydrofurans when conducted in anhydrous
acetonitrile. 86 The reactions are stereospecific, with the E-alcohols generating the
trans and the Z-isomer the cis product. These are 5-endo cyclizations, which are
preferred to 4-exo reactions.
I 2 I
I
I 2
NaHCO 3
HO C H NaHCO 3 HO CH 3 CN O C H
2 5
CH CN O C H C H 2 5
2 5
2 5
3
95% 60%
With the corresponding secondary alcohols, the preferred cyclization is via a confor-
mation with a pseudoequatorial conformation.
2
C H I 2 I C H I NaHCO I
2 5
2 5
NaHCO 3
HO C H 3 C H HO C H O C H
4 9
2 5
H
3
4 9
CH CN 2 5 O C 4 9 C H CH CN 4 9
3
90% 60%
I + R Z
R E
R 1 O
83
B. B. Snider and M. I. Johnston, Tetrahedron Lett., 26, 5497 (1985).
84 C. Neukome, D. P. Richardson, J. H. Myerson, and P. A. Bartlett, J. Am. Chem. Soc., 108, 5559 (1986).
85 A. B. Reitz, S. O. Nortey, B. E. Maryanoff, D. Liotta, and R. Monahan, III, J. Org. Chem., 52, 4191
(1981).
86
J. M. Banks, D. W. Knight, C. J. Seaman, and G. G. Weingarten, Tetrahedron Lett., 35, 7259 (1994); S.
B. Bedford, K. E. Bell, F. Bennett, C. J. Hayes, D. W. Knight, and D. E. Shaw, J. Chem. Soc., Perkin
Trans. 1, 2143 (1999).