Page 340 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 340
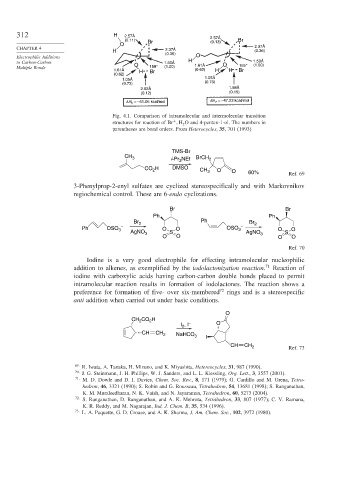
312 H 2.57Å 2.57Å
(0.11) Br (0.13) Br
O
CHAPTER 4 2.37Å 2.37Å
H (0.36)
(0.36)
Electrophilic Additions O
to Carbon-Carbon O 1.50Å H O 1.50Å
(1.00)
Multiple Bonds 159° (1.00) 1.61Å 155°
1.61Å H Br (0.62) H H Br
(0.62)
1.05Å
1.05Å (0.73)
(0.73)
2.02Å 1.98Å
(0.12) (0.15)
ΔH = –51.05 kcal/mol ΔH F = –47.23 kcal/mol
F
Fig. 4.1. Comparison of intramolecular and intermolecular transition
+
structures for reaction of Br H 2 O and 4-penten-1-ol. The numbers in
parentheses are bond orders. From Heterocycles, 35, 701 (1993)
TMS-Br
CH 3 i-Pr NEt BrCH 2
2
CO H DMSO CH 3 O O 60% Ref. 69
2
3-Phenylprop-2-enyl sulfates are cyclized stereospecifically and with Markovnikov
regiochemical control. These are 6-endo cyclizations.
Br Br
Ph Ph
Br 2 Ph Br 2
Ph OSO 3 – AgNO O S O OSO 3 – AgNO O S O
3
O O 3 O O
Ref. 70
Iodine is a very good electrophile for effecting intramolecular nucleophilic
addition to alkenes, as exemplified by the iodolactonization reaction. 71 Reaction of
iodine with carboxylic acids having carbon-carbon double bonds placed to permit
intramolecular reaction results in formation of iodolactones. The reaction shows a
preference for formation of five- over six-membered 72 rings and is a stereospecific
anti addition when carried out under basic conditions.
O
CH CO H
2
2
I , I – O
2
CH CH 2 NaHCO 3 I
CH CH 2
Ref. 73
69
R. Iwata, A. Tanaka, H. Mizuno, and K. Miyashita, Hetereocycles, 31, 987 (1990).
70 J. G. Steinmann, J. H. Phillips, W. J. Sanders, and L. L. Kiessling, Org. Lett., 3, 3557 (2001).
71
M. D. Dowle and D. I. Davies, Chem. Soc. Rev., 8, 171 (1979); G. Cardillo and M. Orena, Tetra-
hedron, 46, 3321 (1990); S. Robin and G. Rousseau, Tetrahedron, 54, 13681 (1998); S. Ranganathan,
K. M. Muraleedharan, N. K. Vaish, and N. Jayaraman, Tetrahedron, 60, 5273 (2004).
72 S. Ranganathan, D. Ranganathan, and A. K. Mehrota, Tetrahedron, 33, 807 (1977); C. V. Ramana,
K. R. Reddy, and M. Nagarajan, Ind. J. Chem. B, 35, 534 (1996).
73
L. A. Paquette, G. D. Crouse, and A. K. Sharma, J. Am. Chem. Soc., 102, 3972 (1980).