Page 341 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 341
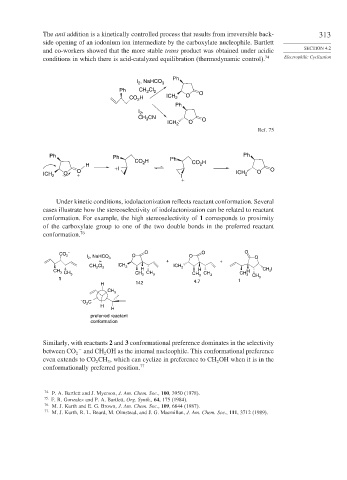
The anti addition is a kinetically controlled process that results from irreversible back- 313
side opening of an iodonium ion intermediate by the carboxylate nucleophile. Bartlett
and co-workers showed that the more stable trans product was obtained under acidic SECTION 4.2
conditions in which there is acid-catalyzed equilibration (thermodynamic control). 74 Electrophilic Cyclization
Ph
, NaHCO
I 2 3
Ph CH 2 Cl 2 O
H ICH 2 O
CO 2
Ph
I ,
2
CH CN O
3
ICH 2 O
Ref. 75
Ph Ph Ph
CO H Ph CO H
H 2 2
+I
O ICH O O
ICH 2 O + I 2
+
Under kinetic conditions, iodolactonization reflects reactant conformation. Several
cases illustrate how the stereoselectivity of iodolactonization can be related to reactant
conformation. For example, the high stereoselectivity of 1 corresponds to proximity
of the carboxylate group to one of the two double bonds in the preferred reactant
conformation. 76
– O O O
CO 2 , NaHCO O O
I 2 3 O
+ +
CH Cl 2 ICH 2 ICH 2
2
H
2
CH 3 CH CH CH H CH CH H CH I
3 3 3 CH 3 3 3 CH
1 4.7 1 3
H 142
CH 3
–
O C
2
H
H
preferred reactant
conformation
Similarly, with reactants 2 and 3 conformational preference dominates in the selectivity
between CO 2 − and CH OH as the internal nucleophile. This conformational preference
2
even extends to CO CH , which can cyclize in preference to CH OH when it is in the
2
3
2
conformationally preferred position. 77
74
P. A. Bartlett and J. Myerson, J. Am. Chem. Soc., 100, 3950 (1978).
75
F. R. Gonzalez and P. A. Bartlett, Org. Synth., 64, 175 (1984).
76 M. J. Kurth and E. G. Brown, J. Am. Chem. Soc., 109, 6844 (1987).
77
M. J. Kurth, R. L. Beard, M. Olmstead, and J. G. Macmillan, J. Am. Chem. Soc., 111, 3712 (1989).