Page 577 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 577
X 551
O CH 2 O CH
PhCH CH X > PhCH CH 2 SECTION 6.3
.
.
.
.
[2 + 2] Cycloadditions
and Related Reactions
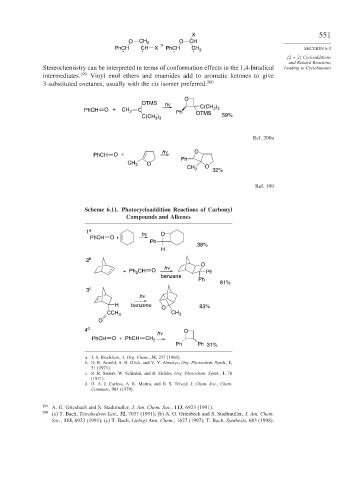
Stereochemistry can be interpreted in terms of conformation effects in the 1,4-biradical Leading to Cyclobutanes
intermediates. 199 Vinyl enol ethers and enamides add to aromatic ketones to give
3-substituted oxetanes, usually with the cis isomer preferred. 200
O
OTMS h ν C(CH )
PhCH O + CH 2 C Ph OTMS 3 3
C(CH ) 59%
3 3
Ref. 200a
h ν O
PhCH O +
Ph
O
CH 3
CH 3 O
32%
Ref. 199
Scheme 6.11. Photocycloaddition Reactions of Carbonyl
Compounds and Alkenes
1 a h ν O
PhCH O +
Ph
38%
H
2 b
O
h ν
CH O
+ Ph 2 Ph
benzene
Ph
81%
3 c
h ν
H benzene O 83%
CCH 3 CH 3
O
4 d O
h ν
PhCH O + PhCH CH 2
Ph Ph 31%
a. J. S. Bradshaw, J. Org. Chem., 31, 237 (1966).
b. D. R. Arnold, A. H. Glick, and V. Y. Abraitys, Org. Photochem. Synth., 1,
51 (1971).
c. R. R. Sauers, W. Schinksi, and B. Sickles, Org. Photochem. Synth., 1,76
(1971).
d. H. A. J. Carless, A. K. Maitra, and H. S. Trivedi J. Chem. Soc., Chem.
Commun., 984 (1979).
199 A. G. Griesbach and S. Stadtmuller, J. Am. Chem. Soc., 113, 6923 (1991).
200
(a) T. Bach, Tetrahedron Lett., 32, 7037 (1991); (b) A. G. Griesbeck and S. Stadtmuller, J. Am. Chem.
Soc., 113, 6923 (1991); (c) T. Bach, Liebigs Ann. Chem., 1627 (1997); T. Bach, Synthesis, 683 (1998).