Page 572 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 572
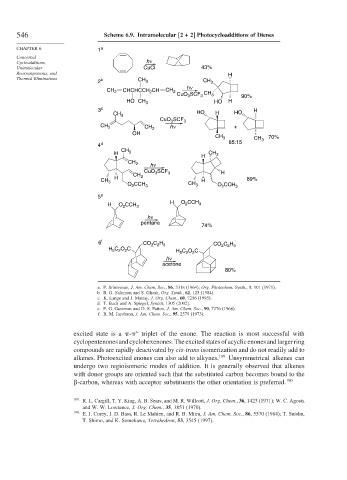
546 Scheme 6.9. Intramolecular [2 + 2] Photocycloadditions of Dienes
CHAPTER 6 1 a
Concerted
Cycloadditions, h ν
Unimolecular CuCl 43%
Rearrangements, and H
Thermal Eliminations b CH
2 3 CH 3
h ν
CH 2 CHCHCCH CH CH 2 CH
2
CuO SCF 3 3 90%
3
HO CH 3 HO H
3 c H
CH 3 HO H HO
CuO SCF 3
3
CH 2 CH 2 h ν +
OH
CH 3 CH 3 70%
4 d 85:15
CH
H 3 CH 3
H
h ν
CH 2
CuO SCF H
CH 3 3
H 2 89%
CH 3 CH H
O CCH 3 3 O 2 CCH 3
2
5 e
H O 2 CCH 3 H O CCH 3
2
h ν
pentane
74%
6 f CO C H CO C H
2 2 5
2 2 5
H C O C H C O C
5 2
2
5 2
2
h ν
acetone
80%
a. P. Srinivasan, J. Am. Chem. Soc., 86, 3318 (1964); Org. Photochem. Synth., 1, 101 (1971).
b. R. G. Salomon and S. Ghosh, Org. Synth., 62, 125 (1984).
c. K. Lange and J. Mattay, J. Org. Chem., 60, 7256 (1995).
d. T. Bach and A. Spiegel, Synlett, 1305 (2002).
e. P. G. Gassman and D. S. Patton, J. Am. Chem. Soc., 90, 7276 (1968).
f. B. M. Jacobson, J. Am. Chem. Soc., 95, 2579 (1973).
excited state is a - triplet of the enone. The reaction is most successful with
∗
cyclopentenonesandcyclohexenones.Theexcitedstatesofacyclicenonesandlargerring
compounds are rapidly deactivated by cis-trans isomerization and do not readily add to
alkenes. Photoexcited enones can also add to alkynes. 189 Unsymmetrical alkenes can
undergo two regioisomeric modes of addition. It is generally observed that alkenes
with donor groups are oriented such that the substituted carbon becomes bound to the
-carbon, whereas with acceptor substituents the other orientation is preferred. 190
189 R. L. Cargill, T. Y. King, A. B. Sears, and M. R. Willcott, J. Org. Chem., 36, 1423 (1971); W. C. Agosta
and W. W. Lowrance, J. Org. Chem., 35, 3851 (1970).
190
E. J. Corey, J. D. Bass, R. Le Mahieu, and R. B. Mitra, J. Am. Chem. Soc., 86, 5570 (1984); T. Suishu,
T. Shimo, and K. Somekawa, Tetrahedron, 53, 3545 (1997).