Page 571 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 571
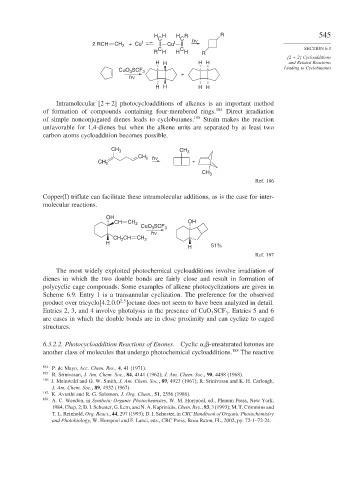
H H H R R 545
C C h ν
2 RCH CH 2 + Cu I Cu I
C C SECTION 6.3
R H H H R
[2 + 2] Cycloadditions
H H H H and Related Reactions
Leading to Cyclobutanes
CuO 3 SCF 3
h ν +
H H H H
Intramolecular 2 + 2 photocycloadditions of alkenes is an important method
of formation of compounds containing four-membered rings. 184 Direct irradiation
of simple nonconjugated dienes leads to cyclobutanes. 185 Strain makes the reaction
unfavorable for 1,4-dienes but when the alkene units are separated by at least two
carbon atoms cycloaddition becomes possible.
CH 3 CH 3
CH 2 h ν
CH 2 +
CH 3
Ref. 186
Copper(I) triflate can facilitate these intramolecular additions, as is the case for inter-
molecular reactions.
OH
CH CH 2 OH
CuO SCF 3
3
h ν
CH CH CH 2
2
H
H 51%
Ref. 187
The most widely exploited photochemical cycloadditions involve irradiation of
dienes in which the two double bonds are fairly close and result in formation of
polycyclic cage compounds. Some examples of alkene photocyclizations are given in
Scheme 6.9. Entry 1 is a transannular cyclization. The preference for the observed
2 5
product over tricyclo[4.2.0.0 ]octane does not seem to have been analyzed in detail.
Entries 2, 3, and 4 involve photolysis in the presence of CuO SCF . Entries 5 and 6
3
3
are cases in which the double bonds are in close proximity and can cyclize to caged
structures.
6.3.2.2. Photocycloaddition Reactions of Enones. Cyclic , -unsaturated ketones are
another class of molecules that undergo photochemical cycloadditions. 188 The reactive
184 P. de Mayo, Acc. Chem. Res., 4, 41 (1971).
185 R. Srinivasan, J. Am. Chem. Soc., 84, 4141 (1962); J. Am. Chem. Soc., 90, 4498 (1968).
186
J. Meinwald and G. W. Smith, J. Am. Chem. Soc., 89, 4923 (1967); R. Srinivasan and K. H. Carlough,
J. Am. Chem. Soc., 89, 4932 (1967).
187 K. Avasthi and R. G. Salomon, J. Org. Chem., 51, 2556 (1986).
188
A. C. Weedon, in Synthetic Organic Photochemistry, W. M. Horspool, ed., Plenum Press, New York,
1984, Chap. 2; D. I. Schuster, G. Lem, and N. A. Kaprinidis, Chem. Rev., 93, 3 (1993); M. T. Crimmins and
T. L. Reinhold, Org. React., 44, 297 (1993); D. I. Schuster, in CRC Handbook of Organic Photochemistry
and Photobiology, W. Horspool and F. Lanci, eds., CRC Press, Boca Raton, FL, 2002, pp. 72-1–72-24.