Page 566 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 566
540
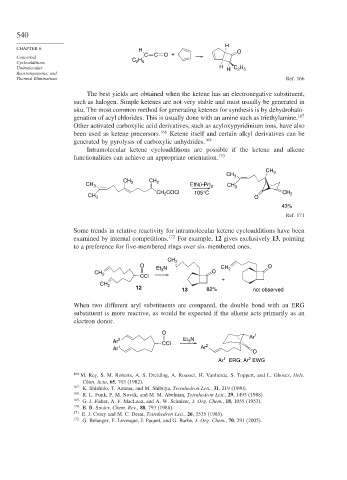
H
CHAPTER 6 H O
C C O +
Concerted H
Cycloadditions, C 2 5
Unimolecular H H C H
2 5
Rearrangements, and
Thermal Eliminations Ref. 166
The best yields are obtained when the ketene has an electronegative substituent,
such as halogen. Simple ketenes are not very stable and must usually be generated in
situ. The most common method for generating ketenes for synthesis is by dehydrohalo-
genation of acyl chlorides. This is usually done with an amine such as triethylamine. 167
Other activated carboxylic acid derivatives, such as acyloxypyridinium ions, have also
been used as ketene precursors. 168 Ketene itself and certain alkyl derivatives can be
generated by pyrolysis of carboxylic anhydrides. 169
Intramolecular ketene cycloadditions are possible if the ketene and alkene
functionalities can achieve an appropriate orientation. 170
CH
CH 3 3
CH CH
CH 3 3 2 EtN(i-Pr) 2 CH 3
CH COCl 105°C CH
CH 3 2 O 2
43%
Ref. 171
Some trends in relative reactivity for intramolecular ketene cycloadditions have been
examined by internal competitions. 172 For example, 12 gives exclusively 13, pointing
to a preference for five-membered rings over six-membered ones.
CH 2
O O
Et N CH 2
3
CH 2 O
CCl
+
CH 2
12 13 82% not observed
When two different aryl substituents are compared, the double bond with an ERG
substituent is more reactive, as would be expected if the alkene acts primarily as an
electron donor.
O Ar 1
3
Ar 2 CCl Et N
Ar 1 Ar 2
O
1
2
Ar ERG; Ar EWG
166
M. Rey, S. M. Roberts, A. S. Dreiding, A. Roussel, H. Vanlierde, S. Toppert, and L. Ghosez, Helv.
Chim. Acta, 65, 703 (1982).
167
K. Shishido, T. Azuma, and M. Shibuya, Tetrahedron Lett., 31, 219 (1990).
168 R. L. Funk, P. M. Novak, and M. M. Abelman, Tetrahedron Lett., 29, 1493 (1988).
169
G. J. Fisher, A. F. MacLean, and A. W. Schnizer, J. Org. Chem., 18, 1055 (1953).
170
B. B. Snider, Chem. Rev., 88, 793 (1988).
171 E. J. Corey and M. C. Desai, Tetrahedron Lett., 26, 3535 (1985).
172
G. Belanger, F. Levesque, J. Paquet, and G. Barbe, J. Org. Chem., 70, 291 (2005).