Page 567 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 567
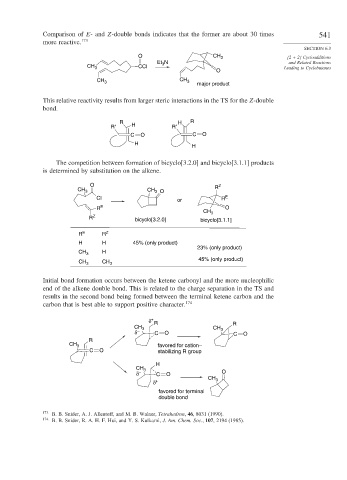
Comparison of E- and Z-double bonds indicates that the former are about 30 times 541
more reactive. 173
SECTION 6.3
O CH 3 [2 + 2] Cycloadditions
Et N and Related Reactions
CH 3 CCl 3 Leading to Cyclobutanes
O
CH
CH 3 3
major product
This relative reactivity results from larger steric interactions in the TS for the Z-double
bond.
R H R
R′ H R′
C O C O
H
H
The competition between formation of bicyclo[3.2.0] and bicyclo[3.1.1] products
is determined by substitution on the alkene.
O Z
CH R
CH 3 3 O
Cl or R E
R E O
CH 3
R Z bicyclo[3.2.0] bicyclo[3.1.1]
R E R Z
H H 45% (only product)
23% (only product)
CH 3 H
45% (only product)
CH
CH 3 3
Initial bond formation occurs between the ketene carbonyl and the more nucleophilic
end of the alkene double bond. This is related to the charge separation in the TS and
results in the second bond being formed between the terminal ketene carbon and the
carbon that is best able to support positive character. 174
δ + R
CH 3 CH R
δ – C O 3 C O
R
CH 3 favored for cation–
C O stabilizing R group
H
CH 3
δ – C O O
δ + CH 3
favored for terminal
double bond
173 B. B. Snider, A. J. Allentoff, and M. B. Walner, Tetrahedron, 46, 8031 (1990).
174
B. B. Snider, R. A. H. F. Hui, and Y. S. Kulkarni, J. Am. Chem. Soc., 107, 2194 (1985).