Page 568 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 568
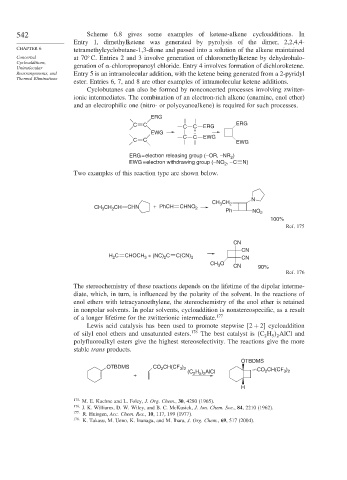
542 Scheme 6.8 gives some examples of ketene-alkene cycloadditions. In
Entry 1, dimethylketene was generated by pyrolysis of the dimer, 2,2,4,4-
CHAPTER 6 tetramethylcyclobutane-1,3-dione and passed into a solution of the alkene maintained
Concerted at 70 C. Entries 2 and 3 involve generation of chloromethylketene by dehydrohalo-
Cycloadditions,
Unimolecular genation of -chloropropanoyl chloride. Entry 4 involves formation of dichloroketene.
Rearrangements, and Entry 5 is an intramolecular addition, with the ketene being generated from a 2-pyridyl
Thermal Eliminations
ester. Entries 6, 7, and 8 are other examples of intramolecular ketene additions.
Cyclobutanes can also be formed by nonconcerted processes involving zwitter-
ionic intermediates. The combination of an electron-rich alkene (enamine, enol ether)
and an electrophilic one (nitro- or polycyanoalkene) is required for such processes.
ERG
C C C C ERG ERG
EWG + –
C C EWG
C C EWG
ERG = electron releasing group (– OR, –NR )
2
EWG = electron withdrawing group (– NO , – C N)
2
Two examples of this reaction type are shown below.
N
CH CH 2
3
CH CH CH CHN + PhCH CHNO 2
3
2
Ph NO 2
100%
Ref. 175
CN
CN
H C CHOCH + (NC) C C(CN) 2 CN
3
2
2
CH O CN 90%
3
Ref. 176
The stereochemistry of these reactions depends on the lifetime of the dipolar interme-
diate, which, in turn, is influenced by the polarity of the solvent. In the reactions of
enol ethers with tetracyanoethylene, the stereochemistry of the enol ether is retained
in nonpolar solvents. In polar solvents, cycloaddition is nonstereospecific, as a result
of a longer lifetime for the zwitterionic intermediate. 177
Lewis acid catalysis has been used to promote stepwise 2 + 2 cycloaddition
of silyl enol ethers and unsaturated esters. 178 The best catalyst is C H AlCl and
2 5 2
polyfluoroalkyl esters give the highest stereoselectivity. The reactions give the more
stable trans products.
OTBDMS
OTBDMS CO CH(CF )
2
3 2
(C H ) AlCl CO CH(CF )
2
3 2
+ 2 5 2
H
175
M. E. Kuehne and L. Foley, J. Org. Chem., 30, 4280 (1965).
176
J. K. Williams, D. W. Wiley, and B. C. McKusick, J. Am. Chem. Soc., 84, 2210 (1962).
177 R. Huisgen, Acc. Chem. Res., 10, 117, 199 (1977).
178
K. Takasu, M. Ueno, K. Inanaga, and M. Ihara, J. Org. Chem., 69, 517 (2004).