Page 569 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 569
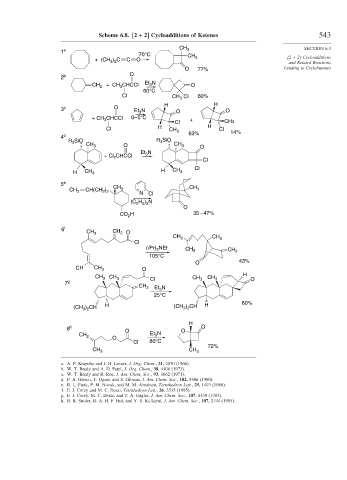
Scheme 6.8. [2 + 2] Cycloadditions of Ketenes 543
CH SECTION 6.3
1 a 3
70°C CH
+ (CH 3 ) 2 C C O 3 [2 + 2] Cycloadditions
and Related Reactions
O 77% Leading to Cyclobutanes
2 b O
CH + CH CHCCl Et N O
3
3
2
60°C
Cl CH 3 Cl 60%
H H
3 c O Et N O O
3
+ CH 3 CHCCl 0– 5°C +
Cl CH3
Cl H CH 3 H Cl 14%
4 d SiO 63%
R 3 SiO R 3
CH 3 O CH 3 O
Et N
CHCCl 3
+ Cl 2
Cl
H CH 3 H CH 3 Cl
5 e CH
CH CH(CH 2 2 3 CH 3
)
2
N Cl
(C H ) N
2 5 3
O
CO H 35 – 47%
2
6 f CH
CH 3 2 O
CH 3 CH 3
Cl
(i Pr) NEt CH 3 CH 2
2
105°C
O 43%
CH CH 3 O
H
CH 3 CH 3 Cl CH 3 CH 3 O
7 g
CH 2 Et N
3
25°C
(CH ) CH H (CH ) CH H 80%
3 2
3 2
H
8 h O O O
3
CH 2 Et N
O
Cl 80°C
72%
CH 3 CH 3
a. A. P. Krapcho and J. H. Lesser, J. Org. Chem., 31, 2030 (1966).
b. W. T. Brady and A. D. Patel, J. Org. Chem., 38, 4106 (1973).
c. W. T. Brady and R. Roe, J. Am. Chem. Soc., 93, 1662 (1971).
d. P. A. Grieco, T. Oguri, and S. Gilman, J. Am. Chem. Soc., 102, 5886 (1980).
e. R. L. Funk, P. M. Novak, and M. M. Abraham, Tetrahedron Lett., 29, 1493 (1988).
f. E. J. Corey and M. C. Desai, Tetrahedron Lett., 26, 3535 (1985).
g. E. J. Corey, M. C. Desai, and T. A. Engler, J. Am. Chem. Soc., 107, 4339 (1985).
h. B. B. Snider, R. A. H. F. Hui, and Y. S. Kulkarni, J. Am. Chem. Soc., 107, 2194 (1985).