Page 574 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 574
548 O O
CHAPTER 6 (CH ) CHCH 2 h ν (CH ) CHCH 2
3 2
3 2
Concerted
Cycloadditions, CH 3
CH 3
Unimolecular
Rearrangements, and Ref. 195c
Thermal Eliminations
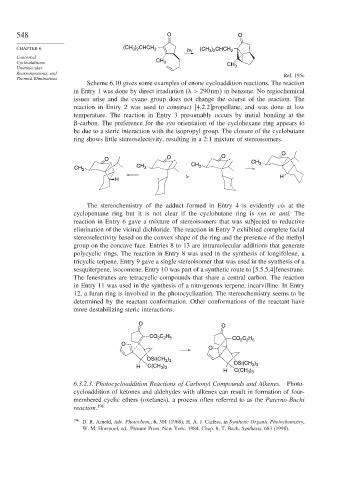
Scheme 6.10 gives some examples of enone cycloaddition reactions. The reaction
in Entry 1 was done by direct irradiation ( > 290nm in benzene. No regiochemical
issues arise and the cyano group does not change the course of the reaction. The
reaction in Entry 2 was used to construct [4.2.2]propellane, and was done at low
temperature. The reaction in Entry 3 presumably occurs by initial bonding at the
-carbon. The preference for the syn orientation of the cyclohexane ring appears to
be due to a steric interaction with the isopropyl group. The closure of the cyclobutane
ring shows little stereoselectivity, resulting in a 2:1 mixture of stereoisomers.
O
O O . O . CH
CH 3
CH 3 3
CH 3
H . > . H
The stereochemistry of the adduct formed in Entry 4 is evidently cis at the
cyclopentane ring but it is not clear if the cyclobutane ring is syn or anti. The
reaction in Entry 6 gave a mixture of stereoisomers that was subjected to reductive
elimination of the vicinal dichloride. The reaction in Entry 7 exhibited complete facial
stereoselectivity based on the convex shape of the ring and the presence of the methyl
group on the concave face. Entries 8 to 13 are intramolecular additions that generate
polycyclic rings. The reaction in Entry 8 was used in the synthesis of longifolene, a
tricyclic terpene. Entry 9 gave a single stereoisomer that was used in the synthesis of a
sesquiterpene, isocomene. Entry 10 was part of a synthetic route to [5.5.5.4]fenestrane.
The fenestranes are tetracyclic compounds that share a central carbon. The reaction
in Entry 11 was used in the synthesis of a nitrogenous terpene, incarvilline. In Entry
12, a furan ring is involved in the photocyclization. The stereochemistry seems to be
determined by the reactant conformation. Other conformations of the reactant have
more destabilizing steric interactions.
O O
CO C H CO C H
2 2 5
O 2 2 5
O
OSi(CH )
3 3
)
H C(CH ) OSi(CH 3 3
3 3
H C(CH )
3 3
6.3.2.3. Photocycloaddition Reactions of Carbonyl Compounds and Alkenes. Photo-
cycloaddition of ketones and aldehydes with alkenes can result in formation of four-
membered cyclic ethers (oxetanes), a process often referred to as the Paterno-Buchi
reaction. 196
196
D. R. Arnold, Adv. Photochem., 6, 301 (1968); H. A. J. Carless, in Synthetic Organic Photochemistry,
W. M. Horspool, ed., Plenum Press, New York, 1984, Chap. 8; T. Bach, Synthesis, 683 (1998).