Page 576 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 576
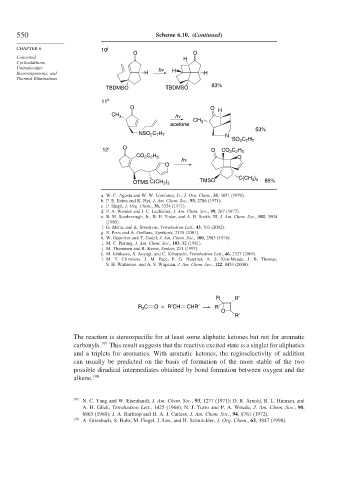
550 Scheme 6.10. (Continued)
CHAPTER 6 10 j
O O
Concerted H
Cycloadditions,
Unimolecular h ν
Rearrangements, and H H H
Thermal Eliminations
TBDMSO TBDMSO 83%
11 k
O O H
CH 3 h ν
CH 3
acetone
53%
NSO C H N
2 7 7
SO C H
2 7 7
12 l O O CO C H
2 2 5
CO C H h ν O
2 2 5
O
C(CH )
3 3
OTMS C(CH ) TMSO 85%
3 3
a. W. C. Agosta and W. W. Lowrance, Jr., J. Org. Chem., 35, 3851 (1970).
b. P. E. Eaton and K. Nyi, J. Am. Chem. Soc., 93, 2786 (1971).
c. P. Singh, J. Org. Chem., 36, 3334 (1971).
d. P. A. Wender and J. C. Lechleiter, J. Am. Chem. Soc., 99, 267 (1977).
e. R. M. Scarborough, Jr., B. H. Toder, and A. B. Smith, III, J. Am. Chem. Soc., 102, 3904
(1980).
f. G. Mehta and K. Sreenivas, Tetrahedron Lett., 43, 703 (2002).
g. E. Piers and A. Orellana, Synthesis, 2138 (2001).
h. W. Oppolzer and T. Godel, J. Am. Chem. Soc., 100, 2583 (1978).
i. M. C. Pirrung, J. Am. Chem. Soc., 103, 82 (1981).
j. M. Thommen and R. Keese, Synlett, 231 (1997).
k. M. Ichikawa, S. Aoyagi, and C. Kibayashi, Tetrahedron Lett., 46, 2327 (2005).
l. M. T. Crimmins, J. M. Pace, P. G. Naternet, A. S. Kim-Meade, J. B. Thomas,
S. H. Watterson, and A. S. Wagman, J. Am. Chem. Soc., 122, 8453 (2000).
R R′
R 2 C O + R′CH CHR′ R
O
R′
The reaction is stereospecific for at least some aliphatic ketones but not for aromatic
carbonyls. 197 This result suggests that the reactive excited state is a singlet for aliphatics
and a triplets for aromatics. With aromatic ketones, the regioselectivity of addition
can usually be predicted on the basis of formation of the more stable of the two
possible diradical intermediates obtained by bond formation between oxygen and the
alkene. 198
197 N. C. Yang and W. Eisenhardt, J. Am. Chem. Soc., 93, 1277 (1971); D. R. Arnold, R. L. Hinman, and
A. H. Glick, Tetrahedron Lett., 1425 (1964); N. J. Turro and P. A. Wriede, J. Am. Chem. Soc., 90,
6863 (1968); J. A. Barltrop and H. A. J. Carless, J. Am. Chem. Soc., 94, 8761 (1972).
198
A. Griesbach, S. Buhr, M. Fiegel, J. Lex, and H. Schmickler, J. Org. Chem., 63, 3847 (1998).