Page 562 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 562
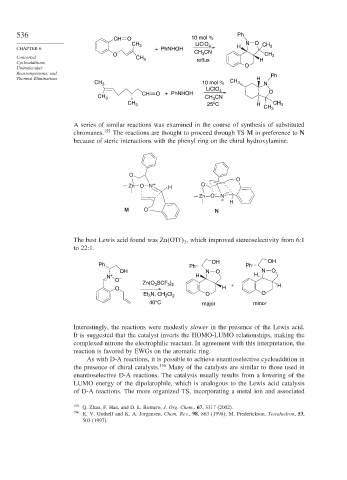
536 Ph
CH O 10 mol %
LiClO N O
CH 3 4 CH 3
CHAPTER 6 + PhNHOH H
3
O CH CN CH 3
Concerted CH 3
Cycloadditions, reflux H
Unimolecular O
Rearrangements, and Ph
Thermal Eliminations H
CH 3 10 mol % CH 3 N
LiClO 4
CH O + PhNHOH O
CH 3 CH CN
3
CH 3 25 C H CH 3
o
CH 3
A series of similar reactions was examined in the course of synthesis of substituted
chromanes. 155 The reactions are thought to proceed through TS M in preference to N
because of steric interactions with the phenyl ring on the chiral hydroxylamine.
O
O
+
Zn O N H O
Zn O – N
+
H
M O N
The best Lewis acid found was Zn OTf , which improved stereoselectivity from 6:1
2
to 22:1.
OH OH
Ph Ph Ph
OH N O N O
N + H H
O –
Zn(O SCF ) +
3
3 2
O H H
Et N, CH Cl 2 O O
2
3
40°C major minor
Interestingly, the reactions were modestly slower in the presence of the Lewis acid.
It is suggested that the catalyst inverts the HOMO-LUMO relationships, making the
complexed nitrone the electrophilic reactant. In agreement with this interpretation, the
reaction is favored by EWGs on the aromatic ring.
As with D-A reactions, it is possible to achieve enantioselective cycloaddition in
the presence of chiral catalysts. 156 Many of the catalysts are similar to those used in
enantioselective D-A reactions. The catalysis usually results from a lowering of the
LUMO energy of the dipolarophile, which is analogous to the Lewis acid catalysis
of D-A reactions. The more organized TS, incorporating a metal ion and associated
155 Q. Zhao, F. Han, and D. L. Romero, J. Org. Chem., 67, 3317 (2002).
156
K. V. Gothelf and K. A. Jorgensen, Chem. Rev., 98, 863 (1998); M. Frederickson, Tetrahedron, 53,
503 (1997).