Page 558 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 558
532 Scheme 6.6 gives some examples of 1,3-DCA reactions. Entry 1 is an addition of an aryl
azide to norbornene. The EWG nitro group is rate enhancing and the reaction occurs
CHAPTER 6 −3 −1 −1
with a rate constant of 6 3×10 M s at 25 C. Owing to steric approach control,
Concerted the product is the exo stereoisomer. Entry 2 involves an acetylenic dipolarophile and
Cycloadditions,
Unimolecular gives an aromatic triazole as the product. Entry 3 is an addition of diazomethane to
Rearrangements, and the dioxolane derivative of acrolein. The reaction is carried out in a closed vessel at
Thermal Eliminations
room temperature. Entry 4 involves a nitrone as the 1,3-dipole. Nitrone cycloadditions
are particularly useful in synthesis because a new carbon-carbon bond is formed and
the adducts can be reduced to -amino alcohols. Nitrile oxides, which are formed by
dehydration of nitroalkanes or by oxidation of oximes with hypochlorite, 151 are also
useful 1,3-dipoles. They are highly reactive, must be generated in situ, 152 and react
with both alkenes and alkynes. The product in Entry 5 is an example in an isoxazole
that was eventually converted to a prostaglandin derivative.
Intramolecular 1,3-dipolar cycloadditions have proven to be especially useful in
synthesis. 153 The products of nitrone-alkene cycloadditions are isoxazolines and the
oxygen-nitrogen bond can be cleaved by reduction, leaving both an amino and hydroxy
function in place. A number of imaginative syntheses have employed this strategy.
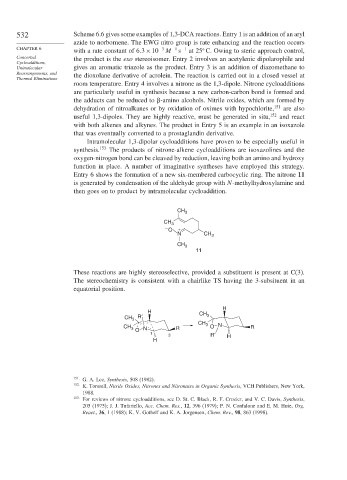
Entry 6 shows the formation of a new six-membered carbocyclic ring. The nitrone 11
is generated by condensation of the aldehyde group with N-methylhydroxylamine and
then goes on to product by intramolecular cycloaddition.
CH 3
CH 3
O +
N CH 3
CH 3
11
These reactions are highly stereoselective, provided a substituent is present at C(3).
The stereochemistry is consistent with a chairlike TS having the 3-subsituent in an
equatorial position.
H
H CH
CH 3 R′ 3
CH
CH 3 O N R 3 O N R
1 R′
3 H
H
151 G. A. Lee, Synthesis, 508 (1982).
152 K. Torssell, Nitrile Oxides, Nitrones and Nitronates in Organic Synthesis, VCH Publishers, New York,
1988.
153
For reviews of nitrone cycloadditions, see D. St. C. Black, R. F. Crozier, and V. C. Davis, Synthesis,
205 (1975); J. J. Tufariello, Acc. Chem. Res., 12, 396 (1979); P. N. Confalone and E. M. Huie, Org.
React., 36, 1 (1988); K. V. Gothelf and K. A. Jorgensen, Chem. Rev., 98, 863 (1998).