Page 559 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 559
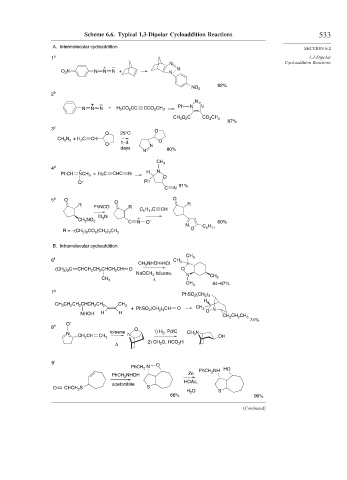
Scheme 6.6. Typical 1,3-Dipolar Cycloaddition Reactions 533
A. Intermolecular cycloaddition
SECTION 6.2
1 a 1,3-Dipolar
N Cycloaddition Reactions
+ –
O 2 N N N N + N N
92%
NO 2
2 b
N
+ –
N N N + H 3 CO 2 CC CCO 2 CH 3 Ph N N
CH 3 O 2 C CO 2 CH 3
87%
3 c
O 25°C O
CH 2 N 2 + H 2 C CH
1–3 O
O N
days 80%
N
CH 3
4 d + N
PhCH NCH 3 +H 2 C CHC N H
O
O – Ph
C N 91%
5 e O O O
R PhNCO R R
C 5 H 11 C CH
Et 3 N +
CH 2 NO 2 –
C N O 60%
N
O C 5 H 11
R = –(CH 2 ) 6 CO 2 (CH 2 ) 3 CH 3
B. Intramolecular cycloaddition
CH 3
6 f CH 3
CH 3 NHOH-HCl
(CH 3 ) 2 C CHCH 2 CH 2 CHCH 2 CH O O
NaOCH 3 toluene, N
CH 3 Δ CH 3
64–67%
CH 3
7 g
PhSO 2 (CH 2 ) 3
H
CH 3 CH 2 CH 2 CHCH 2 CH 2 CH 3
+ PhSO 2 (CH ) 3 CH O CH 3 N
2
NHOH H H O
CH 2 CH 2 CH 3
74%
O –
8 h O
toluene 1) H 2 , Pd/C CH 3 N
N N
+ CH 2 CH CH 2 OH
Δ 2) CH 2 O, HCO 2 H
9 i
PhCH 2 N O
PhCH 2 NH HO
PhCH 2 NHOH Zn
HOAc,
acetonitrile
O CHCH 2 S S
H 2 O S
66% 96%
(Continued)