Page 555 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 555
interpreted in terms of frontier orbital theory. Depending on the relative orbital energies 529
in the 1,3-dipole and dipolarophile, the strongest interaction may be between the
HOMO of the dipole and the LUMO of the dipolarophile or vice versa. Usually SECTION 6.2
for dipolarophiles with EWGs the dipole-HOMO/dipolarophile-LUMO interaction is 1,3-Dipolar
Cycloaddition Reactions
dominant. The reverse is true for dipolarophiles with ERG substituents. In some
circumstances the magnitudes of the two interactions may be comparable. 141 When
HOMO-LUMO interactions control regioselectivity, the reaction is said to be under
electronic control. If steric effects are dominant, the reaction is under steric control.
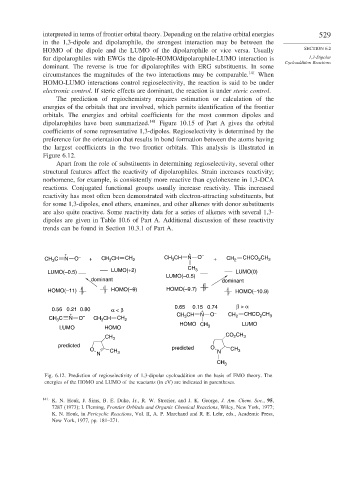
The prediction of regiochemistry requires estimation or calculation of the
energies of the orbitals that are involved, which permits identification of the frontier
orbitals. The energies and orbital coefficients for the most common dipoles and
dipolarophiles have been summarized. 141 Figure 10.15 of Part A gives the orbital
coefficients of some representative 1,3-dipoles. Regioselectivity is determined by the
preference for the orientation that results in bond formation between the atoms having
the largest coefficients in the two frontier orbitals. This analysis is illustrated in
Figure 6.12.
Apart from the role of substituents in determining regioselectivity, several other
structural features affect the reactivity of dipolarophiles. Strain increases reactivity;
norbornene, for example, is consistently more reactive than cyclohexene in 1,3-DCA
reactions. Conjugated functional groups usually increase reactivity. This increased
reactivity has most often been demonstrated with electron-attracting substituents, but
for some 1,3-dipoles, enol ethers, enamines, and other alkenes with donor substituents
are also quite reactive. Some reactivity data for a series of alkenes with several 1,3-
dipoles are given in Table 10.6 of Part A. Additional discussion of these reactivity
trends can be found in Section 10.3.1 of Part A.
+ + –
CH C N O – + CH CH CH 2 CH CH N O + CH 2 CHCO CH 3
2
3
3
3
LUMO(–0.5) LUMO(+2) CH 3 LUMO(0)
LUMO(–0.5)
dominant dominant
HOMO(–11) HOMO(–9) HOMO(–9.7) HOMO(–10.9)
0.56 0.21 0.80 α < β 0.65 0.15 0.74 β > α
+
+ CH CH N O – CH CHCO CH 3
2
CH C N O – CH CH CH 2 3 2
3
3
HOMO CH LUMO
LUMO HOMO 3
CH 3 CO CH 3
2
predicted predicted O
O CH N CH 3
N 3
CH 3
Fig. 6.12. Prediction of regioselectivity of 1,3-dipolar cycloaddition on the basis of FMO theory. The
energies of the HOMO and LUMO of the reactants (in eV) are indicated in parentheses.
141
K. N. Houk, J. Sims, B. E. Duke, Jr., R. W. Strozier, and J. K. George, J. Am. Chem. Soc., 95,
7287 (1973); I. Fleming, Frontier Orbitals and Organic Chemical Reactions, Wiley, New York, 1977;
K. N. Houk, in Pericyclic Reactions, Vol. II, A. P. Marchand and R. E. Lehr, eds., Academic Press,
New York, 1977, pp. 181–271.