Page 551 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 551
O 525
O H
O H O O
O SECTION 6.1
H
O O Diels-Alder Reactions
O
H
5
O
O
O H O O H
O
O
CH OCH O O O
3
2
6 OCH OCH 3 H CH OCH O H
3
2
2
Chiral catalysts (see Section 6.1.6) can also achieve enantioselectivity in IMDA
reactions.
O O
O O
O N
TBDMSO N N H
t -Bu Cu t -Bu
O N TBDMSO
O O
H
96% e.e.
Ref. 131
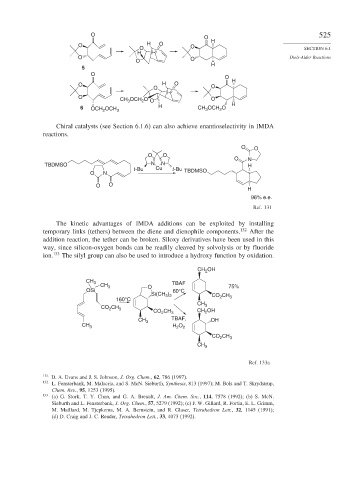
The kinetic advantages of IMDA additions can be exploited by installing
temporary links (tethers) between the diene and dienophile components. 132 After the
addition reaction, the tether can be broken. Siloxy derivatives have been used in this
way, since silicon-oxygen bonds can be readily cleaved by solvolysis or by fluoride
ion. 133 The silyl group can also be used to introduce a hydroxy function by oxidation.
CH OH
2
CH 3 TBAF
CH 3 O 75%
OSi 60°C
Si(CH ) CO CH
3 2
160°C 2 3
CH 3
CO 2 CH 3
CO CH 3 CH OH
2
2
CH 3 TBAF, OH
CH 3 H O 2
2
CO CH 3
2
CH 3
Ref. 133a
131
D. A. Evans and J. S. Johnson, J. Org. Chem., 62, 786 (1997).
132 L. Fensterbank, M. Malacria, and S. McN. Sieburth, Synthesis, 813 (1997); M. Bols and T. Skrydstrup,
Chem. Rev., 95, 1253 (1995).
133 (a) G. Stork, T. Y. Chan, and G. A. Breault, J. Am. Chem. Soc., 114, 7578 (1992); (b) S. McN.
Sieburth and L. Fensterbank, J. Org. Chem., 57, 5279 (1992); (c) J. W. Gillard, R. Fortin, E. L. Grimm,
M. Maillard, M. Tjepkema, M. A. Bernstein, and R. Glaser, Tetrahedron Lett., 32, 1145 (1991);
(d) D. Craig and J. C. Reader, Tetrahedron Lett., 33, 4073 (1992).