Page 552 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 552
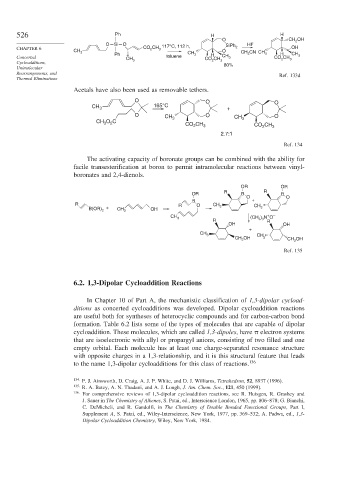
526 Ph H H
O CH 2 OH
O Si O SiPh HF
CHAPTER 6 CO 2 CH 117°C, 112 h, 2 OH
3
CH 3 CH O CH CN CH
Ph 3 H 3 3 H CH
Concerted CH toluene CO CH CH 3 CO CH 3 3
2
Cycloadditions, 3 2 3 80%
Unimolecular
Rearrangements, and Ref. 133d
Thermal Eliminations
Acetals have also been used as removable tethers.
O O O
CH 3 165°C +
O CH O CH O
CH O C 3 CO CH 3 3 CO CH 3
2
3
2
2
2.7:1
Ref. 134
The activating capacity of boronate groups can be combined with the ability for
facile transesterification at boron to permit intramolecular reactions between vinyl-
boronates and 2,4-dienols.
OR OR
R R
OR B B
O O
B +
R R O CH
B(OR) + CH OH 3 CH 3
2 3
+ –
CH 3 (CH ) N O
R 3 3 R
OH OH
+
CH 3 CH
CH OH 3 CH OH
2 2
Ref. 135
6.2. 1,3-Dipolar Cycloaddition Reactions
In Chapter 10 of Part A, the mechanistic classification of 1,3-dipolar cycload-
ditions as concerted cycloadditions was developed. Dipolar cycloaddition reactions
are useful both for syntheses of heterocyclic compounds and for carbon-carbon bond
formation. Table 6.2 lists some of the types of molecules that are capable of dipolar
cycloaddition. These molecules, which are called 1,3-dipoles, have electron systems
that are isoelectronic with allyl or propargyl anions, consisting of two filled and one
empty orbital. Each molecule has at least one charge-separated resonance structure
with opposite charges in a 1,3-relationship, and it is this structural feature that leads
to the name 1,3-dipolar cycloadditions for this class of reactions. 136
134 P. J. Ainsworth, D. Craig, A. J. P. White, and D. J. Williams, Tetrahedron, 52, 8937 (1996).
135 R. A. Batey, A. N. Thadani, and A. J. Lough, J. Am. Chem. Soc., 121, 450 (1999).
136
For comprehensive reviews of 1,3-dipolar cycloaddition reactions, see R. Huisgen, R. Grashey and
J. Sauer in The Chemistry of Alkenes, S. Patai, ed., Interscience London, 1965, pp. 806–878; G. Bianchi,
C. DeMicheli, and R. Gandolfi, in The Chemistry of Double Bonded Functional Groups, Part I,
Supplement A, S. Patai, ed., Wiley-Interscience, New York, 1977, pp. 369–532; A. Padwa, ed., 1,3-
Dipolar Cycloaddition Chemistry, Wiley, New York, 1984.