Page 550 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 550
524
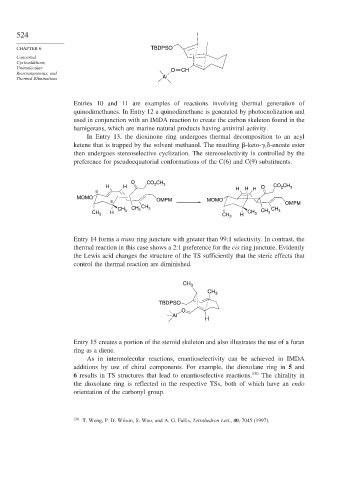
CHAPTER 6 TBDPSO
Concerted
Cycloadditions,
Unimolecular O CH
Rearrangements, and
Thermal Eliminations Al
Entries 10 and 11 are examples of reactions involving thermal generation of
quinodimethanes. In Entry 12 a quinodimethane is generated by photoenolization and
used in conjunction with an IMDA reaction to create the carbon skeleton found in the
hamigerans, which are marine natural products having antiviral activity.
In Entry 13, the dioxinone ring undergoes thermal decomposition to an acyl
ketene that is trapped by the solvent methanol. The resulting -keto- ,
-enoate ester
then undergoes stereoselective cyclization. The stereoselectivity is controlled by the
preference for pseudoequatorial conformations of the C(6) and C(9) substituents.
O CO CH
H H 2 3 H O CO CH 3
2
9 H H
MOMO OMPM MOMO
6 OMPM
CH CH 3 CH 3 CH CH
CH 3 H 3 CH 3 H CH 3 3 3
Entry 14 forms a trans ring juncture with greater than 99:1 selectivity. In contrast, the
thermal reaction in this case shows a 2:1 preference for the cis ring juncture. Evidently
the Lewis acid changes the structure of the TS sufficiently that the steric effects that
control the thermal reaction are diminished.
CH 3
CH 3
TBDPSO
O
Al
H
Entry 15 creates a portion of the steroid skeleton and also illustrates the use of a furan
ring as a diene.
As in intermolecular reactions, enantioselectivity can be achieved in IMDA
additions by use of chiral components. For example, the dioxolane ring in 5 and
6 results in TS structures that lead to enantioselective reactions. 130 The chirality in
the dioxolane ring is reflected in the respective TSs, both of which have an endo
orientation of the carbonyl group.
130
T. Wong, P. D. Wilson, S. Woo, and A. G. Fallis, Tetrahedron Lett., 40, 7045 (1997).