Page 549 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 549
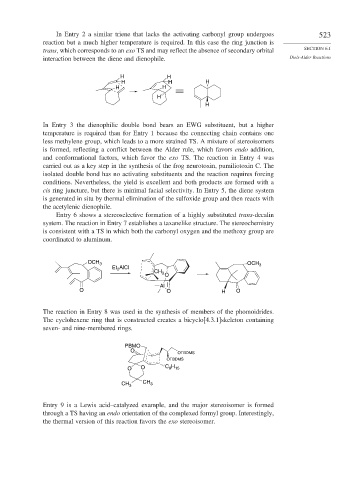
In Entry 2 a similar triene that lacks the activating carbonyl group undergoes 523
reaction but a much higher temperature is required. In this case the ring junction is
trans, which corresponds to an exo TS and may reflect the absence of secondary orbital SECTION 6.1
interaction between the diene and dienophile. Diels-Alder Reactions
H H
H H H
H H
H
H
In Entry 3 the dienophilic double bond bears an EWG substituent, but a higher
temperature is required than for Entry 1 because the connecting chain contains one
less methylene group, which leads to a more strained TS. A mixture of stereoisomers
is formed, reflecting a conflict between the Alder rule, which favors endo addition,
and conformational factors, which favor the exo TS. The reaction in Entry 4 was
carried out as a key step in the synthesis of the frog neurotoxin, pumiliotoxin C. The
isolated double bond has no activating substituents and the reaction requires forcing
conditions. Nevertheless, the yield is excellent and both products are formed with a
cis ring juncture, but there is minimal facial selectivity. In Entry 5, the diene system
is generated in situ by thermal elimination of the sulfoxide group and then reacts with
the acetylenic dienophile.
Entry 6 shows a stereoselective formation of a highly substituted trans-decalin
system. The reaction in Entry 7 establishes a taxanelike structure. The stereochemistry
is consistent with a TS in which both the carbonyl oxygen and the methoxy group are
coordinated to aluminum.
OCH 3 OCH 3
Et 2 AlCl
CH 3 O
Al
O O H O
The reaction in Entry 8 was used in the synthesis of members of the phomoidrides.
The cyclohexene ring that is constructed creates a bicyclo[4.3.1]skeleton containing
seven- and nine-membered rings.
PBMO
O OTBDMS
OTBDMS
8 15
O O C H
CH 3 CH 3
Entry 9 is a Lewis acid–catalyzed example, and the major stereoisomer is formed
through a TS having an endo orientation of the complexed formyl group. Interestingly,
the thermal version of this reaction favors the exo stereoisomer.