Page 560 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 560
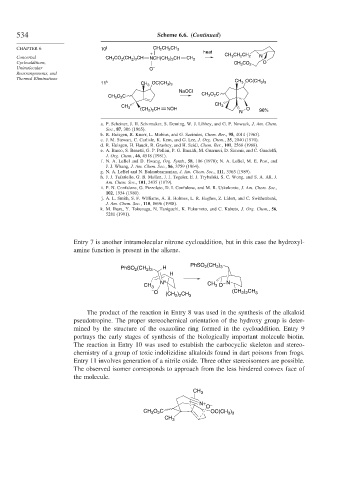
534 Scheme 6.6. (Continued)
CHAPTER 6 j CH 2 CH 2 CH 3
10 heat
+
Concerted CH 3 CO 2 (CH 2 ) 3 CH NCH(CH 2 ) 2 CH CH 2 CH 3 CH 2 CH 2 N
Cycloadditions, CH 3 CO 2 O
Unimolecular O –
Rearrangements, and
Thermal Eliminations
11 k CH 3 OC(CH 3 ) 3 CH 3 OC(CH 3 ) 3
NaOCl
CH 3 O 2 C CH 3 O 2 C
CH 3
CH 3
(CH 2 ) 2 CH NOH O
N 96%
a. P. Scheiner, J. H. Schomaker, S. Deming, W. J. Libbey, and G. P. Nowack, J. Am. Chem.
Soc., 87, 306 (1965).
b. R. Huisgen, R. Knorr, L. Mobius, and G. Szeimies, Chem. Ber., 98, 4014 (1965).
c. J. M. Stewart, C. Carlisle, K. Kem, and G. Lee, J. Org. Chem., 35, 2040 (1970).
d. R. Huisgen, H. Hauck, R. Grashey, and H. Seidl, Chem. Ber., 101, 2568 (1968).
e. A. Barco, S. Benetti, G. P. Pollini, P. G. Baraldi, M. Guarneri, D. Simoni, and C. Gandolfi,
J. Org. Chem., 46, 4518 (1981).
f. N. A. LeBel and D. Hwang, Org. Synth., 58, 106 (1978); N. A. LeBel, M. E. Post, and
J. J. Whang, J. Am. Chem. Soc., 86, 3759 (1964).
g. N. A. LeBel and N. Balasubramanian, J. Am. Chem. Soc., 111, 3363 (1989).
h. J. J. Tufariello, G. B. Mullen, J. J. Tegeler, E. J. Trybulski, S. C. Wong, and S. A. Ali, J.
Am. Chem. Soc., 101, 2435 (1979).
i. P. N. Confalone, G. Pizzolato, D. I. Confalone, and M. R. Uskokovic, J. Am. Chem. Soc.,
102, 1954 (1980).
j. A. L. Smith, S. F. Williams, A. B. Holmes, L. R. Hughes, Z. Lidert, and C. Swithenbank,
J. Am. Chem. Soc., 110, 8696 (1988).
k. M. Ihara, Y. Tokunaga, N. Taniguchi, K. Fukumoto, and C. Kabuto, J. Org. Chem., 56,
5281 (1991).
Entry 7 is another intramolecular nitrone cycloaddition, but in this case the hydroxyl-
amine function is present in the alkene.
PhSO (CH )
PhSO (CH ) H 2 2 3
2 3
2
H
N + CH N
CH 3 3 O
– O (CH ) CH 3 (CH ) CH 3
2 2
2 2
The product of the reaction in Entry 8 was used in the synthesis of the alkaloid
pseudotropine. The proper stereochemical orientation of the hydroxy group is deter-
mined by the structure of the oxazoline ring formed in the cycloaddition. Entry 9
portrays the early stages of synthesis of the biologically important molecule biotin.
The reaction in Entry 10 was used to establish the carbocyclic skeleton and stereo-
chemistry of a group of toxic indolizidine alkaloids found in dart poisons from frogs.
Entry 11 involves generation of a nitrile oxide. Three other stereoisomers are possible.
The observed isomer corresponds to approach from the less hindered convex face of
the molecule.
CH 3
N+ –
O
CH 3 O 2 C OC(CH 3 ) 3
CH 3